Effect of alpha-lipoic acid on rat brain and heart microsomes subjected to oxidative stress induced by ascorbate-Fe++
DOI:
https://doi.org/10.19137/cienvet202426104Keywords:
Alpha-lipoic acid, Oxidative stress, Microsomes, Brain, HeartAbstract
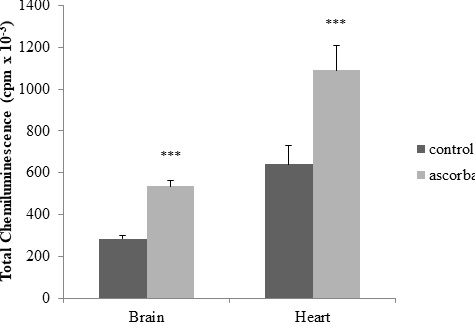
Oxidative stress is caused by the overproduction of reactive oxygen species that generate an imbalance in the cellular antioxidant capacity. In this study, the effect of alpha-lipoic acid on microsomes isolated from rat brain and heart was analyzed by determining chemiluminescence (expressed as counts per minute) and thiobarbituric acid reactive substances. Oxidative stress was induced by subjecting the samples to an ascorbate-Fe++-dependent pro-oxidant system at 37 °C for 120 minutes. The inhibitory effect of alpha-lipoic acid was compared using different concentrations thereof, corresponding to 50, 150 and 250 µg per mg of microsomal protein. Controls were performed simultaneously without the addition of the prooxidant. A significant increase in light emission and malondialdehyde was observed in the microsomes of both organs in the ascorbate-Fe++ group. Analysis of chemiluminescence levels and thiobarbituric acid reactive substances indicated that alpha-lipoic acid acted as an antioxidant that protected rat heart microsomes from damage by lipid peroxidation at all doses tested. In brain microsomes, alpha-lipoic acid was observed to act as an antioxidant only at the 150 µg/ml dose. In the latter case, it will be necessary to test new doses of it to demonstrate the effects on these membranes. In conclusion, alpha-lipoic acid acted as an antioxidant to protect the membranes of both organs against peroxidative damage
Downloads
References
10.1016/j.bbabio.2018.05.019. Epub 2018 May 31. PMID: 29859845.
2. Ferreira CA, Ni D, Rosenkrans ZT, Cai W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018; 11(10):4955-4984. doi:
10.1007/s12274-018-2092-y. Epub 2018 May 26. PMID: 30450165; PMCID: PMC6233906.
3. He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;
44(2):532-553. doi: 10.1159/000485089. Epub 2017 Nov 17. PMID: 29145191.
4. Elsawy H, Al-Omair MA, Sedky A, Al-Otaibi L. Protective effect of α-lipoic acid against α-cypermethrin-induced changes in rat cerebellum. J Chem Neuroanat. 2017; 86:52-
58. doi: 10.1016/j.jchemneu.2017.08.005. Epub 2017 Aug 25. PMID: 28847703.5. Attia M, Essa EA, Zaki RM, Elkordy AA. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants (Basel). 2020; 9(5):359. doi: 10.3390/antiox9050359.
PMID: 32344912; PMCID: PMC7278686.
6. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915-
1928. doi: 10.1083/jcb.201708007. Epub 2018 Apr 18. PMID: 29669742; PMCID: PMC5987716.
7. Tripathi AK, Ray AK, Mishra SK, Bishen SM, Mishra H, Khurana A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev Bras Farmacogn. 2023; 33(2):272-287. doi: 10.1007/s43450-023- 00370-1. Epub 2023 Feb 7. PMID: 36778891; PMCID: PMC9904877.
8. Tutelyan VA, Makhova AA, Pogozheva AV, Shikh EV, Elizarova EV, Khotimchenko SA. Lipoic acid: physiological role and prospects for clinical application. Vopr Pitan. 2019; 88(4):6-11. Russian. doi: 10.24411/0042-8833-2019-10035. Epub 2019 Jul 15. PMID: 31722135.
9. Mohamed WR, Mehany ABM, Hussein RM. Alpha lipoic acid protects against chlorpyrifos-induced toxicity in Wistar rats via modulating the apoptotic pathway.
Environ Toxicol Pharmacol. 2018; 59:17-23. doi: 10.1016/j.etap.2018.02.007. Epub 2018 Feb 23. PMID: 29500983.
10. Khan H, Singh TG, Dahiya RS, Abdel-Daim MM. α-Lipoic Acid, an Organosulfur Biomolecule a Novel Therapeutic Agent for Neurodegenerative Disorders: An
Mechanistic Perspective. Neurochem Res. 2022; 47(7):1853-1864. doi: 10.1007/s11064-022-03598-w. Epub 2022 Apr 21. PMID: 35445914.
11. Kaźmierczak-Barańska J, Boguszewska K, Adamus-Grabicka A, Karwowski BT. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients. 2020 ;12(5):1501. doi: 10.3390/nu12051501. PMID: 32455696; PMCID: PMC7285147.
12. Poletaeva DA, Soldatova YV, Smolina AV, Savushkin MA, Klimanova EN, Sanina NA, Faingold II. The Influence of Cationic Nitrosyl Iron Complex with Penicillamine Ligands on Model Membranes, Membrane-Bound Enzymes and Lipid Peroxidation. Membranes (Basel). 2022; 12(11):1088. doi: 10.3390/membranes12111088. PMID: 36363643; PMCID: PMC9694463.
13. Matsuoka Y, Yamada KI. Detection and structural analysis of lipid-derived radicals in vitro and in vivo. Free Radic Res. 2021; 55(4):441-449. doi:
10.1080/10715762.2021.1881500. Epub 2021 Feb 8. PMID: 33504242.
14. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017; 524:13-30. doi: 10.1016/j.ab.2016.10.021. Epub 2016 Oct 24. PMID: 27789233.
15. Tangen O, Jonsson J, Orrenius S. Isolation of rat liver microsomes by gel filtration. Anal Biochem. 1973; 54(2):597-603. doi: 10.1016/0003-2697(73)90392-8. PMID: 4353369.
16. Gao D, Sakurai K, Chen J, Ogiso T. Protection by baicalein against ascorbic acidinduced lipid peroxidation of rat liver microsomes. Res Commun Mol Pathol Pharmacol. 1995; 90(1):103-14. PMID: 8581335.
17. Venkataramani V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv Exp Med Biol. 2021; 1301:25-40. doi: 10.1007/978-3-030-62026-4_3. PMID: 34370286.
18. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;
186:407-21. doi: 10.1016/0076-6879(90)86134-h. PMID: 2233308.
19. Waterborg JH, Matthews HR. The Lowry method for protein quantitation. Methods Mol Biol. 1994; 32:1-4. doi: 10.1385/0-89603-268-X:1. PMID: 7951715.
20. Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, Murphy MP, Yamamoto M, Winterbourn C. Defining roles of specific reactive oxygen species
(ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022; 23(7):499-515. doi: 10.1038/s41580-022-00456-z. Epub 2022 Feb 21. PMID: 35190722.
21. Mas-Bargues C, Escrivá C, Dromant M, Borrás C, Viña J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch Biochem Biophys. 2021; 709:108941. doi: 10.1016/j.abb.2021.108941. Epub 2021 Jun 17. PMID: 34097903.
22. Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012; 70(5):257-65. doi: 10.1111/j.1753-4887.2012.00476.x. PMID: 22537212.
23. Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li Y, Peng Z. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023; 97(6):1439-1451. doi: 10.1007/s00204-023- 03476-6. Epub 2023 May 2. PMID: 37127681.
24. Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV, Shmanai VV, Kagan VE, Mahal LK, Woerpel KA, Stockwell BR. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018; 14(5):507-515. doi: 10.1038/s41589-018-0031-6. Epub 2018 Apr 2. PMID: 29610484; PMCID:
PMC5899674.
25. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017; 171(2):273-285. doi: 10.1016/j.cell.2017.09.021. PMID: 28985560; PMCID:
PMC5685180.
26. Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev. 2019; 2019:5080843. doi: 10.1155/2019/5080843. PMID: 31737171; PMCID: PMC6815535.
27. Kocaoğlu S, Aktaş Ö, Zengi O, Tufan A, Karagöz Güzey F. Effects of alpha lipoic acid on motor function and antioxidant enzyme activity of nerve tissue after sciatic nerve crush injury in rats. Turk Neurosurg. 2017. doi: 10.5137/1019-5149.JTN.18585- 16.1. Epub ahead of print. PMID: 29044452. 28. Fasipe B, Faria A, Laher I. Potential for Novel Therapeutic Uses of Alpha Lipoic Acid. Curr Med Chem. 2023; 30(35):3942-3954. doi: 10.2174/0929867329666221006115329. PMID: 36201272.
29. Theodosis-Nobelos P, Papagiouvannis G, Tziona P, Rekka EA. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol Biol Rep. 2021;
48(9):6539-6550. doi: 10.1007/s11033-021-06643-z. Epub 2021 Aug 22. PMID: 34420148.
30. Skibska B, Goraca A. The protective effect of lipoic acid on selected cardiovascular diseases caused by age-related oxidative stress. Oxid Med Cell Longev. 2015;
2015:313021. doi: 10.1155/2015/313021. Epub 2015 Apr 8. PMID: 25949771; PMCID: PMC4407629.
31. Basile GA, Iannuzzo F, Xerra F, Genovese G, Pandolfo G, Cedro C, Muscatello MRA, Bruno A. Cognitive and Mood Effect of Alpha-Lipoic Acid Supplementation in a Nonclinical Elder Sample: An Open-Label Pilot Study. Int J Environ Res Public Health. 2023; 20(3):2358. doi: 10.3390/ijerph20032358. PMID: 36767724; PMCID:
PMC9916195.
32. Marmunti ME, Gavazza MB, Palacios A. Effect of alpha-lipoic acid against nonenzymatic peroxidation of rat heart and brain mitochondria. Rev Med Vet Zoot. 2021; 68(1): 11-18. https://doi.org/10.15446/rfmvz.v68n1.97189
Funding This work was supported by the Secretariat of Science and Technology, National University of La Plata (Project 11/V276).
Credits Gavazza M, Marmunti M, Palacios: the three authors worked on conceptualization, validation, formal analysis, research, writing, review, editing and visualization. GM and PA worked on methodology and writing -draft original. PA worked on resources, data curation, supervision and project administration.
Conflict of interest statement
The authors declare that, during the research process, there has been no type of personal, professional or economic interest that could have influenced the judgment and/or actions of the researchers at the time of preparing and publishing this article.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Mariana Beatriz Gavazza, Monica Marmunti, Alejandro Palacios

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Al momento de enviar sus contribuciones, los colaboradores deberán declarar , de manera fehaciente, que poseen el permiso del archivo o repositorio donde se obtuvieron los documentos que se anexan al trabajo, cualquiera sea su formato (manuscritos inéditos, imágenes, archivos audiovisuales, etc.), permiso que los autoriza a publicarlos y reproducirlos, liberando a la revista y sus editores de toda responsabilidad o reclamo de terceros , los autores deben adherir a la licencia Creative Commons denominada “Atribución - No Comercial CC BY-NC-SA”, mediante la cual el autor permite copiar, reproducir, distribuir, comunicar públicamente la obra y generar obras derivadas, siempre y cuando se cite y reconozca al autor original. No se permite, sin embargo, utilizar la obra con fines comerciales.





4.png)


7.png)



