DOI: http://dx.doi.org/10.19137/cienvet202325103
![]() Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No Comercial-
Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons.org.ar/licencias.html
Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No Comercial-
Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons.org.ar/licencias.html
ARTÍCULO DE INVESTIGACIÓN
Canine zoonotic enteroparasites with the “One
Health” approach in Mar del Plata city, Buenos
Aires, Argentina
Enteroparásitos zoonóticos caninos con el
enfoque de “Una Salud” en la ciudad de Mar del
Plata, Buenos Aires, Argentina
Enteroparasitas zoonóticos caninos com a
abordagem "Uma Saúde" nacidade de Mar del
Plata, Buenos Aires, Argentina
Lavallén C1,2, del Río ME3, Allega L4, Denegri GM1,2, Dopchiz MC1,2
1 Laboratorio de Zoonosis Parasitarias, IIPROSAM, FCEyN, UNMdP. Funes 3350, Mar
del Plata (7600), Buenos Ai37373737res, Argentina.
2 CONICET, Argentina.
3 Centro Municipal de Zoonosis, MGP, Hernandarias 10200, Mar del Plata (7600),
Buenos Aires, Argentina.
4 Subprograma de Sensoramiento Remoto, INIDEP. Paseo Victoria Ocampo N°1,
Escollera Norte, Mar del Plata (7600), Argentina.
Corresponding author email: carlalavallen@mdp.edu.ar
ABSTRACT Canine zoonotic parasites have been recognized as a significant
public health problem especially in developing countries with
vulnerable socio-environmental conditions. In the context of “One
Health” the aim of this work was to assess the animal domain by
the evaluation of canine zoonotic enteroparasites associated to a
Parasite Vulnerability Index (PVI) in peripheral (PC) and urban
communities (UC) from Mar del Plata city. The PVI was elaborated
in a previous work as a vulnerability indicator of parasite diseases,
in relation to socio-environmental conditions surveyed in the
communities about the next dimensions: house, sanitation,
hygiene, education and work. A coproparasitological study was
performed to establish the presence and the richness of canine
parasites in the environment in both communities. The PC
evidenced homes with a higher mean number of canine fecal
samples (CFS) with parasites than the UC, showing also the highest
parasite specific richness with helminths and protozoan.
Frequencies of Ancylostomids, Capillariids and Toxocara canis were higher in the PC, but Trichuris vulpis frequencies and the
positive coproantigen test to detect Echinococcus granulosus, were
similar between communities. The PC evidenced association to
variables related to dogs’ ownership (absence of veterinary
attention and not adequate deworming), with the presence of CFS
with parasites and the positive coproantigen test. Families with
medium and high PVI from the PC evidenced a strong association
with the presence of CFS with parasites and also with high
parasitic richness, while families from the UC with low PVI
evidenced CFS without parasites. These results revealed a
vulnerable scenario for the permanence and the transmission of
canine zoonotic parasites in most families from the PC,
highlighting the value of
the socio-environmental features as
predictors of parasitoses.
Keywords: Canine enteroparasitoses; Socio-environmental
conditions; Parasite Vulnerability Index; Peripheral and Urban
Communities; One Health
RESUMEN Los parásitos zoonóticos caninos han sido reconocidos como un
importante problema de salud pública, especialmente en países en
desarrollo con condiciones socioambientales vulnerables. En el
contexto de “Una Salud” el objetivo de este trabajo fue evaluar el
dominio animal mediante la evaluación de enteroparásitos
zoonóticos caninos asociados a un Índice de Vulnerabilidad
Parasitaria (IVP) en comunidades periféricas (CP) y urbanas (CU) de la ciudad de Mar del Plata. El IPV fue elaborado en un trabajo
anterior como un indicador de vulnerabilidad de enfermedades
parasitarias, en relación a las condiciones socioambientales
relevadas en las comunidades sobre las siguientes dimensiones:
vivienda, saneamiento, higiene, educación y trabajo. Se realizó un
estudio coproparasitológico para establecer la presencia y la
riqueza de parásitos caninos en el ambiente de ambas
comunidades. La CP evidenció hogares con mayor número medio
de muestras fecales caninas (MFC) con parásitos que la CU,
mostrando también la mayor riqueza específica parasitaria con
helmintos y protozoos. Las frecuencias de Ancylostomids,
Capillariids y Toxocara canis fueron mayores en la CP, pero las
frecuencias de Trichuris vulpis y la prueba de coproantígeno
positivo para detectar Echinococcus granulosus, fueron similares
entre comunidades. La CP evidenció asociación de variables
relacionadas con la tenencia de perros (ausencia de atención
veterinaria y desparasitación no adecuada), con la presencia de
MFC con parásitos y la prueba de coproantígeno positiva. Las
familias con IVP medio y alto de la CP evidenciaron una fuerte
asociación con la presencia de MFC con parásitos y también con
alta riqueza parasitaria, mientras que las familias de la CU con IVP
bajo evidenciaron MFC sin parásitos. Estos resultados revelaron un
escenario vulnerable para la permanencia y la transmisión de
parásitos zoonóticos caninos en la mayoría de las familias de la CP,
destacando el valor de las características socio ambientales como
predictores de parasitosis.
Palabras clave: Enteroparasitosis caninas; Condiciones socio
ambientales; Índice de Vulnerabilidad de Parásitos; Comunidades
Periféricas y Urbanas, Una Salud
RESUMO Parasitos zoonóticos caninos têm sido reconhecidos como um
importante problema de saúde pública, especialmente em países
em desenvolvimento com condições socioambientais vulneráveis.
No contexto de “One Health” o objetivo deste trabalho foi avaliar o
domínio animal pela avaliação de enteroparasitas zoonóticos
caninos associados a um Índice de Vulnerabilidade Parasitária
(PVI) em comunidades periféricas (PC) e urbanas (UC) de Mar del
Plata cidade. O PVI foi elaborado em trabalho anterior como
indicador de vulnerabilidade a doenças parasitárias, em relação às condições socioambientais levantadas nas comunidades nas
seguintes dimensões: moradia, saneamento, higiene, educação e
trabalho. Um estudo coproparasitológico foi realizado para
estabelecer a presença e a riqueza de parasitas caninos no
ambiente em ambas as comunidades. O PC evidenciou domicílios
com maior número médio de amostras fecais caninas (CFS) com
parasitas do que o UC, apresentando também a maior riqueza
específica parasitária com helmintos e protozoários. As
frequências de Ancilostomídeos, Capillariids e Toxocara canis foram maiores no PC, mas as frequências de Trichuris vulpis e o
teste de coproantígeno positivo para detectar Echinococcus
granulosus, foram semelhantes entre as comunidades. O CP
evidenciou associação com variáveis relacionadas à posse de cães
(ausência de atendimento veterinário e não vermifugação
adequada), com a presença de SFC com parasitas e o teste de
coproantígeno positivo. Famílias com PVI médio e alto do PC
evidenciaram forte associação com a presença de SFC com
parasitos e também com alta riqueza parasitária, enquanto
famílias da UC com baixo PVI evidenciaram SFC sem parasitos.
Esses resultados revelaram um cenário vulnerável para a
permanência e transmissão de parasitoses zoonóticas caninas na
maioria das famílias do PC, destacando o valor das características
socioambientais como preditores de parasitoses.
Palavras-Chave: Enteroparasitoses caninas; Condições
socioambientais; Índice de Vulnerabilidade Parasitária
;
Comunidades Periféricas e Urbanas; Uma Saúde
Of some 1400 species of infectious pathogens of humans, nearly
60% are derived from animal sources, hence the importance of
recognizing the role of livestock, companion animals and wildlife
in the interactions between animals and humans. In that way the
concept of “One Health” (OH) is currently used to describe the
interconnections between people, animals, plants and their shared
environment, and it advocates for increased collaboration among
diverse scientific disciplines in order to mitigate many of the
wicked problems that impact healt1, 2. An important goal would be to identify available information, such as human and animal
morbidity rates from zoonotic diseases, which could serve to
provide decision-makers with a more concrete concept of One
Health’s added value and benefit3.
Zoonotic parasites continue to cause significant morbidity and
mortality mainly to poor and marginalized populations that lack
access to health services and are readily ignored4. Dog feces
harboring infective parasitic forms (larvae, eggs, cysts of helminths
and oocysts of protozoan) are potential sources of environmental
contamination and represent a high risk of infection for the people,
especially in developing countries and communities living with
vulnerable socio-environmental conditions5, 6.
Destabilizing factors consist in those conditions which produce
imbalance in the parasite–host relationship and generate parasite
disease7, 8. These conditions could be explained by the resilience of
some life stages in surviving adverse environmental conditions, or
by the complexity of parasite life cycles some of which involve
people, animals, vectors, and/or transmission through the
environment9. Parasitic infections cannot be solved by eliminating
the parasites, it is also necessary to take control of different
aspects like the improvement of sanitation conditions10. That’s for
socio-environmental features are also considered with a huge
importance in the transmission of parasite zoonoses and they
make sense in the construction of ‘‘One Health’’.
Mar del Plata city (Buenos Aires province, Argentina) has
experienced a sub-urbanization beyond the boundaries of the
main city, reflecting the fast population growth and the high
migration from rural areas to the cities which characterized Latin
America and Argentina11. The expansion on peri-urban areas has
been in a disorderly manner and without planning, with deep
regional contrasts, a negative impact on the environment and a
deteriorating people‘ s life quality as consequences12,13. This
generates an epidemiological framework that is characterized by
poor socio-environmental conditions which make peripheral
populations vulnerable to get parasitoses14-16. In that sense, a
Parasite Vulnerability Index (PVI) was built in a previous study,
taking socio-environmental variables such as: overcrowding, floor
type, drinking water source, wastewater disposal, solid waste
disposal, presence of animals and schooling level. Associations between high PVI and human parasitoses were seen mainly in the
peripheral community compared with the urban community15.
The previous study included the association between the human
and the environmental domains, being necessary to approach the
animal domain through the infection status of zoonotic
enteroparasites and its relation with disease risk factors in the
community17. The aims of the present study were: i) to research the
presence of canine zoonotic enteroparasites in the same urban
communities from Mar del Plata city studied before, and ii) to
analyze associations between canine parasitic infections and the
PVI previously built in both communities.
The study was conducted in two communities from Mar del Plata
city, General Pueyrredon district, located on the southeast coast of
Buenos Aires Province, Argentina (38°S; 57°33’W). The peripheral
community (PC) belonged to the neighborhood of ‘‘Santa Rosa del
Mar’’, located 14 km southwest from downtown. The estimated
population was 500 inhabitants, and it was characterized by
precarious housing with limited access to public services. The
urban community (UC) was constituted by families whose children
attend an urban kindergarten, and by other families who wanted
to join the study. They lived in neighborhoods located in the urban
area, and most of them counted with all the public services. These
communities were participants of the previous study of zoonoses
parasites affecting children in relation with socio-environmental
variables, assessing the human and the environmental domains of
the ‘‘One Health’’ approach.
A descriptive and cross-sectional epidemiological study was
carried out in both communities. Epidemiological surveys were
designed to measure housing variables through information
regarding structural qualities, amenities, and family features. A
total of 108 families from the PC agreed to participate in the study,
while the population from the UC was formed by 43 families.
A Parasite Vulnerability Index (PVI) previously elaborated was
used as a vulnerability indicator to parasite diseases, in relation to
the socio-environmental conditions surveyed about the next
dimensions: house, sanitation, hygiene, education and work. PVI
was classified as low (1–1.6), medium (1.7–2.2) and high (2.3–2.7)
(15).
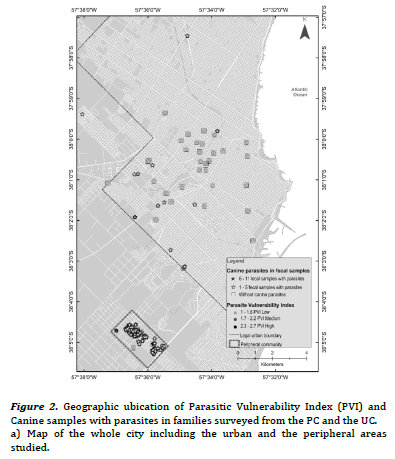
Thematic maps of punctual implantation were made from the
primary data referencing each sampling unit (homes surveyed)
and overlapping thematic layers such as PVI, canine fecal samples
with parasites and specific richness. The different shades of grey
indicate the condition of vulnerability with respect to the PVI. The
thematic maps obtained were elaborated with the geographical
information system QGIS version 2.14.
A coproparasitological study was performed to establish the existence of canine parasites in the environment. Three hundred and six (306) fresh canine fecal samples (CFS) from the PC and 46 from the UC were collected in the houses surveyed, processed through the modified Sheather's flotation technique, and microscopically examined18. Parasitic loads were estimated by means of the number of eggs (helminths) or cysts (protozoan) in fields of 100x and 400x, respectively, because stool samples were taken and kept with formaldehyde 10% (v/v). In case of helminths, worm burdens were estimated 2 per field (light), 3–6 (moderate) and higher than 7 (heavy). A burden of more than five cysts per field was considered high for protozoan19.
Coproantigen test was performed for detection of the definite host of E. granulosus. Following a methodology of coproantigen determination for E. granulosus, 109 CFS from the PC and 27 from the UC were processed20. The samples were sent to the Laboratory of Parasitology (Cathedra of Microbiology and Parasitology, Medicine School, Comahue National University).
Data analysis was performed using Info Stat and MedCalc version 4.6b21,22. The use of epidemiological files generated a lot of categorical variables; some of them were analyzed through the Multiple Correspondence Analysis (MCA), which operates on the Chi-square deviations matrix. This method measures the combination of modalities that have more inertia, contributing most to reject the hypothesis of independence between two variables. Comparisons of proportions were used to establish differences between frequencies of canine parasites, and between the communities (significance level of 95–99%). The species richness (number of parasite species/families) in each community, and the Sørensen similarity coefficient (degree of enteroparasites similarity in percentage terms) were also calculated23.
With the collaboration of veterinarians from the Municipal Centre of Zoonoses, several talks were performed with the PC, giving advice about good practices in raising farm animals and pets, then parasitized dogs were deworming. Families from the UC with parasitized dogs were suggested to visit a veterinarian.
The overall frequencies of CFS with parasites were 83.3% (n= 255/306) in the PC and 45.6% (n= 21/46) in the UC. In relation to houses surveyed the 91.1% (n= 82/90) from the PC presented canine parasites, while in the UC was the 40.7% (n= 11/27). The mean number of CFS with parasites per home was statistically significantly higher in the PC than the UC (PC: 2.83 ± 2.12 vs. UC: 0.8 ± 1.12; t= 6.32; P < 0.0000; CI= 1.38 – 2.62). Sørensen similarit y coefficient was 53.3%, and the specific richness was 11 in the PC and 4 in the UC. Helminths were identified in CFS from both communities and protozoan only in the PC; their respective frequency and dominance were assessed (Table 1). Among the parasites found the highest frequencies belonged to Ancylostomids and T. vulpis. The frequencies of Ancylostomids and Capillariids were statistically significantly higher in the PC and T. vulpis frequencies were similar in both communities. Due the complex morphological characteristics among the eggs of the Capillariidae family, some CFS were further analyzed in other study, taking account morphological and molecular features, arriving to the identification of eggs assigned to Eucoleus aerophilus, E. boehmi and Calodium hepaticum24.
Table 1. Parasites found in canine fecal samples from peripheral
and urban communities.
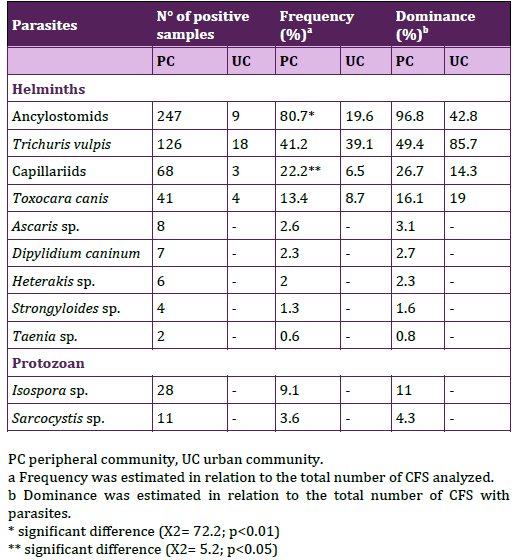
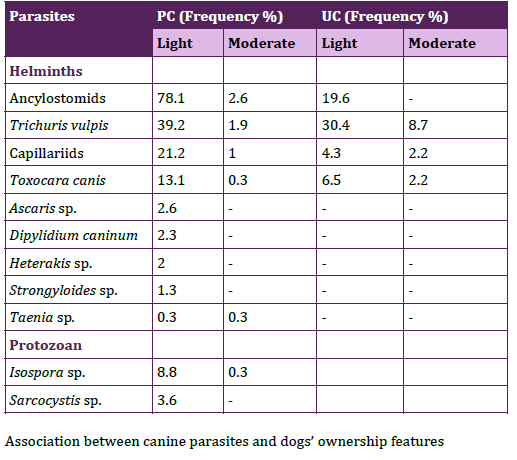
The coproantigen test for E. granulosus, did not show significative difference between frequencies of positive samples in the PC and the UC (16.5%, n= 18/109; 22.2%, n= 6/27). Parasitic loads of helminths were mainly light in both communities (PC 91.7%, n=253; UC 90.5, n=21) and parasitic loads of protozoan were light in the PC (100%, n=36). Frequencies of CFS with the parasitic loads for each parasite were shown in Table 2.
Table 2. Frequencies of canine fecal samples with light and moderate
parasitic loads in peripheral and urban communities.
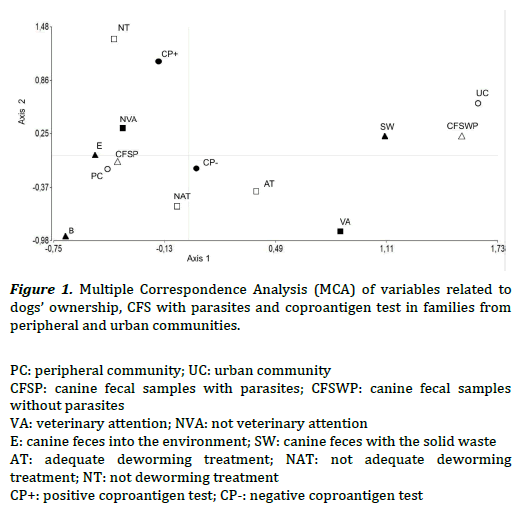
The MCA evidenced association between variables related to dogs’ ownership, the existence of CFS with parasites and the coproantigen test. In Figure 1, the left quadrant showed that families from the PC evidenced association to: CFS with parasites (CFSP), the habit of leaving canine feces into the environment (E), the absence of veterinary attention (NVA), not adequate deworming treatment (NAT) or the absence of it (NT), and positive coproantigen test (CP+). In the right quadrant families from the UC showed association to: CFS without parasites (CFSWP), the habit of disposing the canine feces with solid waste (SW), veterinary attention (VA), adequate deworming treatment (AT) and negative coproantigen test (CP-). Statistically significant association were seen between: CFSP/NAT when they were stratified by communities (v2 Cochran–Mantel–Haenszel = 7.48, P< 0.05); CFSP/NVA (X2 MV-G2= 8.91, P< 0.01; OR = 0.24, 0.09–0.6) and CFSP/E (X2 MV-G2= 28.68, P< 0.01).
The PVI previously developed for PC and UC, had shown statistically significant differences between frequencies of families with high, medium and low PVI (high PVI: 38.9% PC vs. 2.3% UC, X2 = 18.5, P < 0.01; medium PVI: 55.5% PC vs. 4.6% UC, X2 = 30.9, P< 0.01; low PIV: 5.6% PC vs. 93% UC, X2 = 106.8, P < 0.01)15. Figure 2(a) evidenced most families with low PVI without canine parasites from UC, while figure 2(b) revealed association mainly between families with medium and high PVI and the presence of 1- 5 fecal samples with parasites in the PC. Furthermore, some of them reported 6-11 fecal samples with parasites. Figure 3(a) evidenced mainly families from the UC with low PVI and without canine parasites or with a parasitic richness of 1-4 species, while in figure 3(b) was observed an important association between hig or medium PVI and a parasitic richness of 1-4 and 5-9 species in families from the PC. In that sense the analysis between PVI and parasitic richness evidenced also similar associations than those that occurred between PVI and fecal samples with parasites.
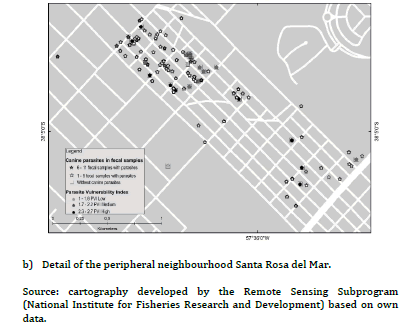
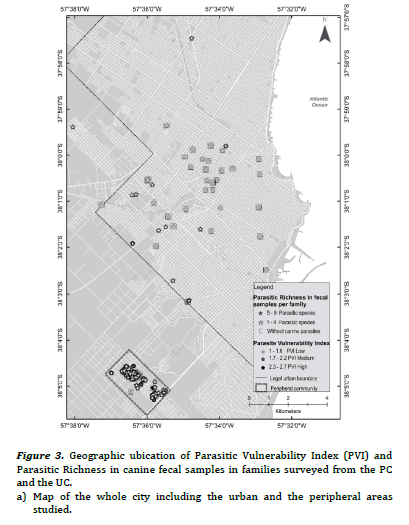
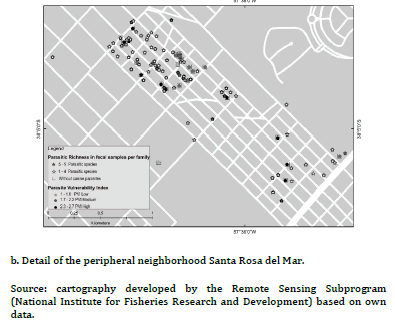
The three OH domains should be evaluated to approach zoonotic
canine enteroparasites, because they significantly affect humans
and domestic animals’ health, and the infective structures are
transmitted through the ecosystem.
The previous study developed in the same communities from Mar
del Plata city, have been focused on the human and environmental
domains, integrating several socio-environmental features to
address the con-causal factors of toxocarosis and
enteroparasitoses affecting children15. This study evidenced that
deficient socio-environmental conditions made children more
vulnerable to get enteroparasitoses and toxocarosis in the PC than
in the UC, where the PVI was higher. After the analysis of these
domains, the present study attempted to approach the animal
domain through the study of canine parasites in association with
the same PVI.
More than sixty parasite species are related to canines and among
them several enteroparasites can occasionally infect humans
causing zoonotic parasitoses5. The two communities involved in
this study, evidenced CFS with parasites, but the overall frequency
was highest in the PC. The high mean number of CFS with parasites
per home in the PC revealed an important source of environmental
contamination with parasitic forms of zoonotic potential, also
reinforced for the major specific richness of parasites. The maps of
punctual implantations exposed medium and high PVI in most of
these families, which lead to the idea that people from the PC was
more vulnerable to be infected with a wide range of canine
parasites than the UC. These results were concomitant with the
existence of not appropriated habits of dogs’ ownership in the
families of the PC, like the habit of leaving canine feces into the
environment, the absence of veterinary attention and deworming.
On the other hand, a strong association was seen between the
absence of canine parasites and appropriated habits of dogs’ ownership in the UC where low PVI was prevalent. In contrast with
these results, a study performed in Bahía Blanca (Buenos Aires
province), developed an assay to evaluate the occurrence of canine
parasites in relation to a Quality Life Index (QLI), finding no
differences in the overall parasite frequencies between the areas
studied with very high, high and low QLI25. The present work
revealed a possible context for the transmission of canine zoonotic
parasites in both communities, requiring more attention in the PC
where the poor habitational quality, the socio-economic situation,
the lack of education and hygiene and the habits of raising pets and
poultry, become into the con-causal factors which allow the
establishment, the permanence, and the dissemination of zoonotic
parasites 7.
The assessment of canine enteroparasites in many countries has
been very heterogeneous, with frequencies from 12% to 96%. The
higher frequencies (66% to 96%) belonged to countries with
deficient socio-economic conditions added to cultural habits that
propend to the parasite transmission26,27. Other countries with
better economic incomings and sanitary conditions showed the
lowest frequencies (12,5% to 40%)28-30. Several studies developed
in Argentina have evidenced environmental contamination with
canine intestinal parasites with heterogeneous results: Ushuaia
(32,5%), Neuquén (37,9%), Chubut (46,6%), Buenos Aires (52,4%), Salta (77,4%), and Bahía Blanca (87%) among others6,31,32.The occurrence of canine intestinal parasites in the PC and the UC,
was similar to the countries with disadvantaged and advantaged
socio-economic conditions respectively. The result from the PC
was between the highest frequencies found in Argentina and also
higher than those reported in peri-urban populations with similar
socio-environmental features in the cities of La Plata (Buenos Aires
province) and Santa Rosa (La Pampa province)33.
In the present study both communities showed CFS with helminths
but only in the PC were observed protozoan. About helminths the
dominant parasites were Ancylostomids in the PC while in the UC
was T. vulpis. The PC showed higher frequencies of Ancylostomids
and Capillariids than the UC, and also evidenced helminths like
Ascaris sp., D. caninum, Heterakis sp., Strogyloides sp. and Taenia
sp. which were absent in the UC. The finding of E. granulosus was
similar in the communities. These results agreed with the low
parasites similarity between the communities and the high specific
richness in the PC. International studies have reported
ancylostomids and T. canis between the most common
geohelminths, followed by Giardia sp. as the usual protozoan, and
Sarcocystis sp. and Isospora sp. also reported in the PC 26, 27-30, 32.
Similar studies from Argentina have shown a lower specific
richness, agreeing with the same protozoan and the geohelminths A. caninum, T. vulpis and T. canis, as the most frequently found
parasites.
The dominance of these parasites could be related to the huge
resistance of the eggs to extreme environmental conditions being a
continuous source of re-infections, and to their ability to evade the
host immune response34. Furthermore, the transmission of
ancylostomids through the per-cutaneous via is more likely in an
environment with high areas of soil and grass as occurred in the
PC, where the development of the filariform larvae can take place.
This situation, reinforced by the presence of feces with moderate
parasitic loads in the area, could increase the transmission of
ancylostomids and the occurrence of the cutaneous larva migrans
syndrome in humans. Regarding T. vulpis, cases of visceral larva
migrans and intestinal infections in humans have been reported35,36.
Both communities were exposed to this parasite, however the
parasitic load was more relevant within the UC, lightening the
importance of a correct dog’s deworming and feces’ disposal.
T. canis produces toxocarosis, a usual helminth neglected disease
in industrialized countries, which affects several organs in its
presentation as visceral larva migrans, and also could cause ocular
and brain toxocarosis37. A review based on the meta-analysis of
250 eligible studies of seroprevalence conducted worldwide,
pointed out a toxocarosis seroprevalence rate of 22.8% (19.7– 26.0%) in the American region38. Potential risk factors associated
with seropositivity included male gender; living in a rural area;
young age; close contact with infected dogs, cats or soil;
consumption of raw meat; drinking and/or irrigating with
untreated water37,38. Eggs of T. canis have been found in studies
performed in urban and peripheral areas from Mar del Plata
city39,40. The previous study conducted in these communities,
showed a statistically significant higher proportion of seropositive
children to Toxocara from the PC (55%), in comparison with the
UC (8.5%). In addition, associations were observed between
seropositivity and variables such as contact with infected dogs, not
adequate hand washing, and moderate and hypereosinophilia in
children from the PC. Most of these children belonged to families
with medium and high PVI15. The existence of T. canis IgG could
indicate past infestations, enhanced for the high frequency of CFS
with this parasite mainly in the PC. Even though the presence of T.
canis was more relevant in the PC, the moderate load observed in
the UC stablished the risk to develop some of the toxocarosis
presentations also in this community.
Within the Capillaridae family, eggs of E. aerophilus, E. bohemi and
C. hepaticum were diagnosed in the CFS analysed. Reports of
Eucoleus sp. are scarce in Argentina. This could be related to the
misdiagnosis due to confusion with morphologically similar
trichuroid eggs, and the lack of knowledge about the species of
Eucoleus in this geographical area. Epidemiological studies
developed on the beach and in peripheral neighbourhoods at local
level, showed low frequencies of CFS with eggs assigned to this
genus6,40. The finding of E. boehmi in the PC is interesting because
this parasite is a neglected and underestimated cause of upper
respiratory disease in dogs41. Furthermore, a unique case of
respiratory capillariosis attributable to E. bohemi has been
reported in the country42. On the other hand, E. aerophilus has been
deeply described as parasitizing the lower respiratory tract of
dogs, and also could cause zoonotic disease in humans43. This
situation took importance in both communities due the presence of the parasite, nevertheless, the transmission risk increased in the
PC where the frequency was higher. Regarding C. hepaticum, it
could cause spurious infections through the ingestion of the
definitive host, principally rodents and the release of eggs in the
feces44.
Heterakis sp. is a genus that parasitized mainly birds and
occasionally rodents45. The existence of Heterakis sp. in the CFS
from the PC, evidenced the real possibility of parasite-host new
associations when the context is appropriated7. Raising poultry
such as geese and chickens without adequate veterinary
assistance, added to the presence of rodents due to the open
dumps in the PC, would generate this context.
Taenia sp./Echinococcus sp., indistinguishable between them by
coprological examination, has been reported in the Patagonia
region of Argentina with frequencies between 0.31% - 12.6%
which was related to the endemism of the cystic echinococcosis in
the region6,31. A previous sampling performed in Gral. Pueyrredon
district also evidenced this kind of eggs46. In the present study the
frequency of samples with Taenia sp. was low, which could be
associated with the poor sensitivity of the flotation technique to
recover tenids’ eggs. The presence of these eggs evidenced that
dogs were fed with raw meat, and the low and moderate loads
would indicate an important liberation of eggs to the environment,
increasing the infection risk and the possible development of cystic
echinococcosis. The existence of tenids’ eggs in two families from
the PC which used to race pigs, added to the cystic echinococcosis
case reported in a child from one of them, and the positive
coproantigen test found in the community, pointed out the
importance of the con-causal factors to the establishment of this
zoonoses47.
Isospora sp. and Sarcocystis sp. found in the PC, could mean a
source of infection for humans since they can persist in the
environment for long periods of time depending on the climate
conditions, contaminating sources of water and vegetables. These
coccids affect mainly children and immunocompromised people
with diarrhea, nausea, anorexia, among other symptoms48.
Strongyloides stercoralis is a common soil-transmitted helminth
that infects humans, primates, cats and also canids, therefore presents both environmental and zoonotic sources of transmission
to humans49. Molecular studies performed on S. stercolaris from
humans and dogs, revealed that both hosts could share the
parasite populations50. Taking account that it has been diagnosed
in canids’ feces in the country and in the region under study and
eggs assigned to Strongyloides sp. were observed in canine feces
from the PC, dogs could be a possible reservoir for zoonotic
infection in this community40, 51.
Regards Ascaris sp. the presence of eggs in the feces would indicate
a coprophagic behavior of dogs, in case the structures would have
belonged to A. lumbricoides52.
The WHO (2012) argues that most infectious diseases, parasitic
zoonoses included, affect mainly poor and marginalized
populations which have no access to health services. Studies
carried out in Argentina gave account of the epidemiological
scenario to the development of canine zoonotic parasitoses, built
up on the vulnerable socio-environmental conditions and deficient
hygiene practices of the populations52. The results of the present
study revealed a vulnerable scenario for the permanence and the
transmission of canine zoonotic parasites in most families from the
PC, highlighting the value of the socio-environmental features as
predictors of parasitoses, as the human cystic echinococcosis, and
other new parasitic associations16. The animal domain addressed in
this work, added to the human and environmental domains deeply
studied in the previous work, offered valuable information into the“One Health” approach to generate a global synergism for all
aspects of health care for humans, animals and the environment.
These tools will alert the authorities about the epidemiological
context for taking decisions to mitigate the effect of canine
intestinal parasitoses on public health.
In the context of ‘‘One Health’’ the results of this work evidenced a
higher vulnerability in the peripheral community to be exposed to
several canine zoonotic parasites. This context added to the
previously results about enteroparasitoses and toxocarosis in
children, pointed out that the Parasite Vulnerability Index was a
useful tool to identify families with deficient socio-environmental
conditions, and reinforced the knowledge of the environmental domain which is essential to understand the transmission of
human and animal parasite diseases.
This work was conducted with support from the National
University of Mar del Plata (grants EXA 764/16, EXA 827/17) and
the Consejo Nacional de Investigaciones Científicas y Técnicas
(CONICET) (grant PIP N° 0115). The Health Department from
General Pueyrredon district collaborated with the study
development.
1. Leboef A. Making Sense of One Health: Cooperating at the Human- Animal-Ecosystem Health Interface. 2011. Health and Environment Reports n° 7. Available: http://onehealthinitiative.com/publications/ IFRI_ifrihereport7alineleboeuf.pdf.
2. One Health Commission Why One Health? 2016 Available https://www.onehealthcommission.org/en/why_one_health/ accessed.
3. Sinclair JR. Importance of a One Health approach in advancing global health security and the Sustainable Development Goals. Rev. Sci. Tech.2019; 38(1), 145-154.
4. World Health Organization Technical Report Series, Research priorities for zoonoses and marginalized infections. Technical report of the TDR Disease Reference Group on Zoonoses and Marginalized Infectious Diseases of Poverty. Report n° 971.2 012. Available: https://apps.who.int/iris/bitstream/handle/10665/75350/WHO_TRS_ 971_eng.pdf.
5. Macpherson CNL.Human behaviour and the epidemiology of parasitic zoonosis. Int. J. Parasitol. 2005; 35(11-12), 1319-1331.
6. Soriano SV, Pierangeli NB, Roccia I, Bergagna HF, Lazzarini LE, Celescinco A, et al. A wide diversity of zoonotic intestinal parasites infects urban and rural dogs in Neuquén, Patagonia, Argentina. Vet. Parasitol. 2010; 167(1), 81-85.
7. Denegri G. Fundamentación epistemológica de la parasitología.2008, EUDEM, Mar del Plata, 111 pp.
8. Yannarella F. Breves consideraciones sobre parasitismo animal. In: Denegri, G., (Ed.), Elogio de la sabiduría. Ensayos en homenaje a Mario Bunge en su 95 aniversario. 2014. EUDEBA, Mar del Plata, pp 323-335.
9. Gibbs EPJ. The evolution of One Health: a decade of progress and challenges for the future. Vet. Rec. 2014; 174(4), 85-91.
10. Denegri GM. Cestodosis de herbívoros domésticos de la República Argentina de importancia veterinaria, 2001, Editorial Martín, Mar del Plata, 111 pp.
11. Dávila, JD. Tan cerca de la ciudad y tan lejos de las tuberías: la gobernabilidad en el agua y el saneamiento periurbano. In: Aguilar, A.G., Escamilla, I. (Eds.), Periferia urbana, deterioro ambiental y reestructuración metropolitana. 2009, Porrúa Editores, Ciudad de México, pp 1-31.
12. Lucero P Población y poblamiento del Partido de General Pueyrredón: la combinación entre tiempo y espacio en la sociogeografía local. In: Velázquez, G., Lucero, P., Mantobani, J.(Eds.), Nuestra Geografía Local: población, urbanización y transformaciones socioterritoriales en el Partido de General Pueyrredón, Argentina, 1975-2000. Grupo de Estudios Sobre Población y Territorio, Departamento de Geografía, Facultad de Humanidades, 2004, UNMdP, Mar del Plata, pp 37-76.
13. Zulaica L, Celemín JP. Análisis territorial de las condiciones de habitabilidad en el periurbano de la ciudad de Mar del Plata (Argentina), a partir de la construcción de un índice y de la aplicación de métodos de asociación espacial. Rev. Geogr. Norte Gd. 2008; 41, 129- 146.
14. Aveni SM. Geografía de la Salud y Calidad de Vida: un análisis de la Condición Sanitaria en Mar del Plata. In: Lucero, P. (Ed.), Territorio y Calidad de Vida, una mirada desde la Geografía Local. 2008, EUDEM, Mar del Plata, pp. 229-251.
15. Lavallén CM, Brignani B, Riesgo K, Rojas A, Colace G, Biscaychipi M, et al. Enteroparasitoses and toxocarosis affecting children from Mar del Plata city, Argentina. Ecohealth 2017; 14(2), 219-233.
16. Lavallén CM, Scioscia NP, Kifer M, Denegri G, Dopchiz MC. La periferia como confluencia de la ruralidad, el urbanismo y las parasitosis: acerca de un caso de echinococcosis quística como ejemplo de detección y predicción de factores de desequilibrio. Ludus Vitalis 2018; 26(49), 61- 74.
17. Schurer JM, Mosites E, Li C, Meschke S, Rabinowitz P. Community-based surveillance of zoonotic parasites in a ‘One Health’ world: A systematic review. One Health 2016; 2, 166-174.
18. Méndez O. Acta Bioquímica Clínica Latinoamericana. Federación Bioquímica de la Provincia de Buenos Aires, 1998, Buenos Aires, 163 pp.
19. Ertug S, Karakas S, Okyay P, Ergin F, Oncu S. The effect of Blastocystis hominis on the growth status of children. Med. Sci. Monit.2007; 13(1), 40 -43.
20. Pierangeli N, Soriano SV, Roccia I, Bergagna HFJ, Lazzarini LE, Celescinco A, et al. Usefulness and validation of a coproantigen test for dog echinococcosis screening in the consolidation phase of hydatid control in Neuquén, Argentina. Parasitol. Int.2010; 59(3), 394-399.
21. Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat versión 2014. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Córdoba, Argentina. URL http://www.infostat.com.ar
22. Schoonjans F. 1993. MedCalc Versión 4.16b – Windows 95.
23. Bush AO, Laffertz KD, Lotz JM, Shostak W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997; 83(4), 575- 583.
24. Lavallén CM, Petrigh RS, Fugassa MH, Denegri GM, Dopchiz MC. First morphological and molecular analysis of Eucoleus boehmi like eggs in dogs from Argentina. Parasitol. 2018; Res. 117(7), 2351-2357.
25. La Sala LF, Leiboff A, Burgos JM, Costamagna SR. Spatial distribution of canine zoonotic enteroparasites in Bahía Blanca, Argentina. Rev. Argent. Microbiol. 2015; 47(1), 17-24.
26. Gingrich EN, Scorza AV, Clifford EL, Olea-Popelka FJ, Lappin MR. Intestinal parasites of dogs on the Galapagos Islands. Vet. Parasitol. 2010; 169(3-4), 404-407.
27. Beiromvand M, Akhlaghi L, Massom SHF, Meamar AR, Motevalian A, Oormazdi. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Prev. Vet. Med. 2013; 109(1-2), 162- 167.
28. Little SE, Johnson EM, Lewis D, Jaklitsch RP, Payton ME, Blagburn BL. Prevalence of intestinal parasites in pet dogs in the United States. Vet. Parasitol.2009; 166(1-2), 144-152.
29. Joffe D, Van Niekerk D, Gagné F, Gilleard J, Kutz S, Lobingier R. The prevalence of intestinal parasites in dogs and cats in Calgary, Alberta. Can. Vet. J.2011; 52(12), 1323-1328.
30. Gillespie S, Bradbury RS. A Survey of Intestinal Parasites of Domestic Dogs in Central Queensland. Trop. Med. Infect. Dis. 2017; 2(60), 1-10.
31. Sánchez Thevenet P, Jensen O, Mellado I, Torrecillas C, Raso S, Flores ME. Presence and persistence of intestinal parasites in canine fecal material collected from the environment in the Province of Chubut, Argentine Patagonia. Vet. Parasitol.2003; 117(4), 263-269.
32. Cociancic P, Deferrari G, Zonta ML, Navone GT. Intestinal parasites in canine feces contaminating urban and recreational areas in Ushuaia (Argentina). Vet. Parasitol. Reg. Stud. Reports. 2020; 21(2020), 100424.
33. Gamboa MI, Navone GT, Orden AB, Torres MF, Castro LE, Oyhenart EE. Socio-enviromental conditions, intestinal parasitic infections and nutritional status in children from a suburban neighborhood of La Plata, Argentina. Acta Trop. 2011; 118(3), 184-189.
34. Traversa D. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasit. Vectors2011; 4, 32.
35. Dunn JJ, Columbus ST, Aldeen WE, Davis M, Carroll KC. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002; 40(7): 2703.
36. Márquez Navarro A, García Bracamontes G, Álvarez Fernández BE, Ávila Caballero LP, Santos Aranda I, Díaz Chiguer DL. Trichuris vulpis (Froelich, 1789) Infection in a Child: A Case Report. Korean J. Parasitol. 2012; 50(1), 69-71.2704.
37. Strube C, Raulf MK, Springer A, Waindok P, Auer H. Seroprevalence of human toxocarosis in Europe: A review and meta-analysis. Adv. Parasitol. 2020; 109, 375-418.
38. Rostami A, Riahi SM, Holland CV, Taghipour A, Khalili-Fomeshi M, Fakhri Y. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019: 13(12), e0007809.
39. Riva E, Sardella N, Hollmann P, Denegri G. Relevamiento coproparasitológico de aceras y calles de la ciudad de Mar del Plata, Argentina. Rev. Vet. 2006; 17(2), 72-76.
40. Bravo NK. Evaluación de la Contaminación Parasitaria Ambiental en el barrio periférico Nuevo Golf (Partido de General Pueyrredon, Buenos Aires, Argentina). 2012. Tesis de grado. Facultad de Cs. Exactas y Naturales, Universidad Nacional de Mar del Plata, 58 pp.
41. Alho AM, Mouro S, Pisarra H, Murta A, Lemos M, Gomes L. First report of Eucoleus boehmi infection in a dog from Portugal. Parasitol.2016; Res. 115, 1721-1725.
42. González PJ, Taba E, González G, Guendulain C, Caffaratti M, Bessone A. et al. Primera comunicación de la parasitación de un canino con Eucoleus boehmi en Argentina. Redvet. 2014; 15(6), 1-11.
43. Traversa D, Di Cesare A, Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit. Vectors2010; 3:62
44. Queiroga Gonçalves A, Ascaso C, Santos I, Taquita Serra P, Rebouças Julião G, Puccinelli Orlandi P.Calodium hepaticum: household clustering transmission and the finding of a source of human spurious infection in a community of the Amazon region. PLoS Negl. Trop. Dis.2012; 6(12), e1943.
45. Paramasvaran S, Sani RA, Hassan L, Hanjeet K, Krishnasamy M, Jeffery J. Endo-parasite fauna of rodents caught in five wet markets in Kuala Lumpur and its potential zoonotic implications. Trop. Biomed.2009; 26(1), 67-72.
46. Lavallén CM, Dopchiz MC, Lobianco E, Hollmann P, Denegri GM. Intestinal parasites of zoonotic importance in dogs from the district of General Pueyrredón, Buenos Aires, Argentina. Rev. Vet.2011; 22(1), 19- 24.
47. Lavallen CM, Pons M, Mercuri E, Ortolani V, Scioscia N, Hollmann P. Atypical cystic echinococcosis in a young child. Pediatr. Infect. Dis. J.2015; 34(2), 226.
48. Huiza A, Espinoza Y, Rojas R, Sevilla C, Alva P. Detección de coccidios en niños asintomáticos mediante esporulación de muestras fecales. An. Fac. Med.2004; 65(4), 1-3.
49. Eslahi AV, Hashemipour S, Olfatifar M, Houshmand E, Hajialilo E, Mahmoudi R. Global prevalence and epidemiology of Strongyloides stercoralis in dogs: a systematic review and meta-analysis for human disease emergence. Parasit. Vectors. 2022; 15, 21.
50. Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017; 11(8), e0005752.
51. Semenas L, Flores V, Viozzi G, Vázquez G, Pérez A, Ritossa L. Helmintos zoonóticos en heces caninas de barrios de Bariloche (Río Negro, Patagonia, Argentina). Rev. Argent. Parasitol.2014; 2(2), 22-27.
52. Gamboa MI, Kozubsky LE, Costas ME, Garraza M, Cardozo MI, Susevic ML.Asociación entre geohelmintos y condiciones socioambientales en diferentes poblaciones humanas de Argentina. Rev. Panam. Salud Pública.2009; 26(1), 1-8.
Fecha de recibido: 25/10/2022
Fecha de aceptado para su publicación: 05/12/2022