 Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No
Comercial-Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons. org.ar/licencias
Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No
Comercial-Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons. org.ar/licenciasDOI: http://dx.doi.org/10.19137/cienvet-201921104
 Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No
Comercial-Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons. org.ar/licencias
Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No
Comercial-Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons. org.ar/licencias
ARTÍCULO DE INVESTIGACIÓN
Temporal and spatial immunolocalization of osteopontin in the repair of orthopaedic bone defects treated with demineralized bone matrix
Inmunolocalización temporal y espacial de osteopontina en la reparación de defectos óseos ortopédicos tratados con matriz ósea desmineralizada
Audisio, S.A.1; Vaquero, P.G.1; Verna E.C.1; Cristofolini, A.L.2; Merkis, C.I.2
1Faculty of Veterinary Science, National University of La Pampa, La Pampa, Argentina.
2Electronic Microscopic Area, Agronomy and Veterinary Faculty, National University of Río
Cuarto, Córdoba, Argentina
Correo electrónico
: saudisio@vet.unlpam.edu.ar
Summary: Osteopontin (OPN) is the most abundant non-collagen protein in
the bone matrix, where it fulfils the function of cellular adhesion and
biomineralization. In the present work, the authors report the temporal
and spatial localization of OPN during the repair of experimental
orthopaedic bone defects treated with demineralized bone matrix
(DBM) processed by the authors. 30 rabbits were used, which were
given an orthopaedic bone defect of critical size in one of the radiuses,
which was filled with DBM. The rabbits were euthanized in groups of 5
individuals at days 7, 15, 21, 30, 60 and 150. Histological cuts were immunomarked
to establish the spatial and temporal immunomarcation
of OPN. The histological cuts were observed with an optic microscope
with which histological images were captured and analysed with the
ImageJ software. The image analysis allowed the authors to establish
the optic density (OD) and the integrated optic density (IOD). The
data was analysed with the ANOVA and Fischer LSD tests. At day 7, the
presence of OPN was observed only in the DBM particles, where the
OD was 0.08 and the IOD was 1.64; at day 15, OPN marked different
sites of collagen condensations and cells contained in the interior of
the matrix. In this period the OD was 0.096 and the IOD, 9.26. At days
21 and 30, the OPN immunosignalled osteocytes, osteoblasts, osteoclasts
and hypertrophic chondrocytes in the bone trabeculae adjacent to the ossification zones. At day 21 the OD was 0.17 and IOD 6.22. At
day 30, the OD was 0.14 and the IOD 2.52. At days 60 and 150, OPN
was evenly distributed in the new bone matrix with an OD: 0.10 and
IOD: 0.48, and OD: 0.35 and IOD: 3.80, respectively. The OD and IOD
showed significant differences (p<=0.05) between days 7, 15, 21 and
30; and there was no difference at days 60 and 150 (p=0.05). OPN was
found in the DBM particles: it increased the optic densities at day 15
and it diminished at day 60, after which it increased the OD and IOD
again until day 150. It was established that the OPN immunoexpressed
during the repair process in indifferentiated cells, osteoprogenitor
chondrocytes and osteoblasts. The variation of OD and IOD allowed
the authors to establish that the greatest degree of immunoexpression
of OPN was at day 15 after repair initiated. On the other hand, the increase
registered between days 60 and 150 post treatment was due to
the biomineralization of the bone matrix.
Key words: Rabbit ; Bone ; Osteopontin ; Inmunomarcation ; Demineralized
bone matrix
Resumen: La osteopontina (OPN) es la proteína no colágena que más abunda
en la matriz ósea, donde cumple funciones de adhesión celular y
biomineralización. En el presente trabajo se informa la localización
temporal y espacial de la OPN durante la reparación de defectos óseos
ortopédicos tratados con matriz ósea desmineralizada (MOD). Se emplearon
30 conejos a los que se les practicó un defecto óseo ortopédico
de tamaño crítico en uno de sus radios. Los defectos se rellenaron con
MOD obtenida según protocolo previamente informado. Los conejos
fueron sacrificados en grupos de 5 a los 7, 15, 21, 30, 120 y 150 días
de los cuales se recuperaron los defectos para identificar las estructuras
histológicas y establecer la inmunomarcación espacial y temporal
de OPN. Se realizaron cortes histológicos de los defectos que se tiñeron
con hematoxilina y eosina (HE) e inmunomarcaron según técnica
inmunohistológica. Los cortes inmunomarcados se observaron en un
microscopio óptico de donde se capturaron imágenes histológicas a 20X para analizarlas con el software ImageJ y establecer la densidadóptica (DO) y densidad óptica integrada (DOI). Los datos se analizaron
con un test ANOVOA y LSD Fisher. A los 7 días se observó la presencia
de OPN solo en las partículas de MOD donde la DO: 0,109 y DOI:
3587,043; a los 15 días OPN marcaba distintos sitios de condensaciones
colágenas y células en su interior, en este período DO fue 0,096 y
la DOI: 10593,08. A los 21 y 30 días OPN señalaba trabéculas óseas,
osteocitos, osteoblastos, osteoclastos y condrocitos hipertróficos inmediatos
a las zonas de osificación, la DO fue 0,134 y DOI 14.639,7.
A los 120 y 156 días OPN se encontraba uniformemente distribuida
por la matriz del hueso nuevo con una DO: 0,0104; DOI: 4160,96 y DO:
0,081 y DOI 8878,9 respectivamente. Las DO y DOI mostraron diferencias
significativas (p<=0,05) entre los 7, 15, 21 y 30 días y no existió diferencia a los días 120 y 150 días (p=0,05). OPN se halló presente
en las partículas de MOD e incrementó las densidades ópticas a los 15,
21 y 30 días como producto del metabolismo celular posibilitando la
adhesión celular y luego interviniendo en la biomineralización.
Palabras claves: Conejo ; Hueso osteopontina ; Inmunomarcación
matriz ósea desmineralizada
Demineralized bone matrix (DBM) is used in orthopaedic surgery
as a bone substitute to repair wide defects due to its osteoinductive
nature.(1) It is constituted by the organic elements which constitute the
bone matrix, among them collagen type I, bone morphogenic protein
(BMP), osteocalcin (OC), osteopontin (OPN), bone sialoprotein (BSP)
and osteonectin(2,3)
OPN is the most abundant non-collagen protein in the extracellular
matrix.(4) It plays a role in various physiological and molecular processes,
such as the stimulation of cell-cell adhesion, the rise of extracellular
cell-matrix communication,(5,6,7)it stimulates the early differentiation
of the osteoblasts,(8)it promotes the migration of immune and
tumoral cells, and it also diminishes cellular death and makes biomineralization
of extracellular matrix possible.(9,10)
The aim of the present article is to establish the spatial and temporal
presence of OPN during the repair process of orthopaedic bone defects treated with DBM processed following a protocol previously
informed by the authors.
The experiments were done following the norms for care of the
Faculty of Veterinary Sciences of the National University of La Pampa.
Thirty sexually mature male and female rabbits were used, which
were kept in individual cages and fed with a balanced formula and
ad-libitum water. After the rabbits became accustomed to the living
conditions the experiment began.
The rabbits were sedated with 0,1 mg/kg of diazepam (Diazepam®,
Lab Zoovet, Arg), 0,01 mg/kg IM of acepromacina (Acedan®, Lab
Holliday, Arg) and were anaesthesized with 40 mg/kg IM of ketamina
(ketamina 50®, Lab Holliday, Arg). In each animal one of the thoracic
limbs was prepared to be operated in aseptic conditions. The radial
diaphysis was approached through the dorsal side of the forearm
where a defect was created equivalent to twice the diameter of the
diaphysis,(11) which meant that it would not heal spontaneously in the
animal’s life.
The defects were filled with demineralized bone matrix which was
processed following a protocol previously informed by the authors.(12) Briefly, the technique consisted in using diaphysis of the long bones of
rabbits whose soft tissue was extracted at 4°C. The diaphysis was fragmented
until particles of 100 to 750 μm were obtained. Lipids were
extracted from the fragmented bone with a chloroform: methanol solution
and then it was demineralized with chloridric acid (HCl) 0.6N.
After stabilizing the pH in 7.0, it was preserved in ethylic alcohol 95° at 4°C until used.
After filling the defects, the extensor muscles were sutured with
poligalactine (Vicryl®, Ethicon, USA) and the subcutaneous tissue
and skin were sutured following routine/usual techniques. After the
surgery, the rabbits received 1.000.000 IU of bencilpeniciline and dihidroestreptomicine
(Dipenisol®, Lab. Bayer, Arg) and nonsteroid
antiinflamatory, Ketofen 0.1 mg/kg (Kalmavet®, Lab Vetanco, Arg) for
3-5days.
The rabbits were divided into six groups according to a specific time
of euthanasia at 7, 15, 21, 60 and 150 days post-operatively in groups
of 5 animals. The defined region of interest was the entire orthopaedic
defect. The samples were fixed with buffered saline formaldehyde,
they were dehydrated with batteries of alcohols of growing graduation,
to be included in paraffin. Histological cuts corresponding to the
provoked and treated defects were done of approximately 4 μm with
microtome (Microtom) and 3-4 cuts were placed on each microscope
slide. The histological cuts were immune marked OPN by means of an
immunohistochemical technique.
Once the paraffin was removed from the cuts, they were rehydrated
and treated with hydrogen peroxide at 3% (v/v), then they were
washed with PBS and later they were incubated with the antibody for
OPN (AKm2A1: sc-21742; Santa Cruz Biotechnology, Inc, USA) during
1 hour at room temperature in a wet chamber. Then, they were washed
with phosphate buffer saline solution (PBS pH 7.2) and incubated
during 20 minutes with the second antibody with biotin (made up by
anti-rabbit, anti-mouse and anti-goat immunoglobulin with biotin).
After being washed again with PBS were treated with the complex
streptavidine combined with peroxidase (LSAB®+Systems HRP, Dako
Cytomation). After the period of incubation, they were washed and
treated with the solution of cromogen substratum 3,3’-daiminobenzidina
(DAB). Then the cuts were contrasted with Mayer hematoxiline,
washed with ammonium hydroxide solution, dehydrated in a battery
of alcohol of growing concentration and mounted with Entellan
(Merck, Alemania).
The expression of OPN was established positive when the extracellular
and cytoplasm of osteoblast, osteocyte and osteoclast was dyed
brown.
Images were taken from the immune-marked cuts with a 200X magnification
using an Axiophot optic microscope (Carl Zeis, Germany) fit
to a digital camera Powershot (G6, 7.1 megapixeles, Canon INC, Japan)
which used the software AxioVision 4.6.3 (Carl Zeis, Germany). The
images corresponded to the new DBM particles, osteochondroid tissue,
of the ossification phase and of the new trabecular bone and mature
bone. The images were analysed following the methodology previously
informed by Vasconcellos et al., (2014)(13)
The immune-markedness of OPN was quantified calculating the
optic density (OD) and the integrated optic density (IOD). To calculate
the OD and IOD the images of the histological cuts were loaded
in the software ImageJ 1.49b (Media Cybernetics, USA). This turned the brown colour of the expression of OPN into grey in a scale that
extends from 0 to 255 (0 corresponding to white and 255 to black).
The optic density was calculated using the formula: OD = Log10 (TI
II); the TI is the transmitted intensity (equivalent to 255) and the II
is the incident intensity, the media of the grey in the image analysed
by the software. The integrated optic density (IOD) is obtained from
the product between the OD and the surface unit expressed in square
microns (μ2) occupied by the expression of OPN, given the formula IOD
= (OD *AREA). The result of both formulas was expressed in arbitrary
units (AU).
The obtained data was loaded in the software Infostat(14) with which
the descriptive statistics was carried out. The medias of each period of
study was analyzed with ANOVA and Fisher LSD test to compare multiple
groups. The statistical signification was defined as p<0.05
The histological studies carried out on the obtained samples following
the postsurgical temporal sequence showed a complete repair
of the defects from 60 days on. The histological and immunohistochemical
observations corresponding to each period of study are described
below.
At day 7 post-treatment, OPN was distributed homogenously in the
matrix, with greater intensity in the central channels of the osteons
and osteocytes lacunae (Fig. 1). The OD was 0.08 (±0.09; min: 0.008-
max: 0.50) and IOD 1.64 (±2.11; min: 0.05-max: 7.37 (Table 1) (Fig. 2).
At day 15 post-surgery, OPN was found in the DBM particles and in
the MC particles that surrounded the former (Fig.1b). In the ossification
sites the OPN was immunoexpressed in the extracellular matrix,
where the transition from cartilaginous matrix to bone matrix was
evident (Fig.1c). It was immunodetected in the hypertrophic chondrocytes
near the areas of ossification and in the edges of the chondroplasts
and chondrocels. It was also immunomarked in the pre-osteoblasts
that proliferated and they differentiated into osteoblasts (Fig.4).
In the bone spiculas, it was immunodistributed in a heterogeneous way
in the matrix, with greater intensity in the surfaces surrounding the
osteocytes included in the matrix. In the spaces constituted between
the spiculas it immunoexpressed in the cytoplasm and cellular membranes
of the preosteoblasts, osteoblasts and osteoclasts (Fig.3A). The OD of the OPN in the studied period was 0.20 (±0.09; min: 0.11-max:
0.33) while the IOD was 9.26 (±11.92; min: 0.97-max: 32.60) (Table 1)
(Fig. 2)
At day 21, OPN was immunodetected in the ossification sites where
it was found in the cytoplasm of the hypertrophic chondrocytes adjacent
to the new bone and in the edges of the chondroplasts that contained
them. In the guideline spiculas, OPN was present in the extracellular
matrix, the osteoid, the osteocytes, osteoplasts and osteoclasts.
In the erosion lines, the osteoclasts expressed OPN in the cytoplasm,
in the cuboidal osteoblasts and in the osteoid (Fig. 4A). In the new
bone spiculas, OPN was immunomarked in the extracellular matrix,
osteoblast, osteoclasts and osteocytes. In the intertrabecular spaces
OPN was found in the preosteoblasts and osteoblasts (Fig. 4B). In this
repair phase the OD was 0.17 (±0.05; min: 0.11-max:0.28) while the
IOD was registered in 6.22 (±3.22; min:2.83-max:13.78) (Table 1).
After 30 days, OPN was found in the same histological sites reported
at day 21. In the cartilaginous tissue, it was found in some hypertrophic
chondrocytes near the ossification site. In the ossification places
there were immunomarked chondroplasts, others were fused and
contained osteoblasts which were also immunomarked OPN (Fig. 5).
OPN was also the constitutive element of the bone trabecules, in which
it presented heterogenous densities of greater optic density in the osteocytes
and in the matrix surrounding them. At day 30, the OD was
0.140 (min: 0.082-max: 0.284) and the IOD, 2.522 (min: 0.086-max:
8.074) (Table 1).
At day 60 post DBM implant, OPN was found distributed in a heterogeneous
way in the lamellar bone, in the matrix of the trabecula. In
the lamellar bone it was also found in the osteocytes and in the matrix
which constituted the lamellae. In the trabecular bone, it was found
in the surface over which there were morphologically flat osteoblasts
(Fig.6). The OD was 0.10 (±0.07; min: 0.01-max: 0.21) and the IOD
0.48 (±0.60; min: 0.01-max: 1.61) (Table 1).
At day 150, OPN was distributed in the bone matrix where it appeared
in circular deposits coinciding with the concentric lamillae of the
osteons (Fig. 7).
The ANOVA and LSD Fisher contrast analysis for OPN showed statistically
significant differences (p>0.05) at days 7, 21 and 150.
The
same statistical analysis for IOD showed that there were statistically
significant differences (p>0.05) of the AU registered at day 15 with
respect to days 7, 21 and 60.
The expression of OPN in the DBM particles showed that the demineralization
process did not remove the matrix. Its permanence had the benefit
that it allowed the mesenchymal cells and osteoclasts adhesion to the
surfaces. The authors could not establish if the values of these parameters
were significant, since they did not find any previous publications registering
OD or IOD values. However, both types of density were significantly
inferior to the registered since day 15, 21 and 30, as the present cells
synthesized OPN during those periods, raising its presence and intensity
markation.
From day 15 DBM particles, mesenchymal tissue condensations and
bone spiculas were observed in the interior of the defects. The condensations
generated new cartilage which ossified and finally repaired the defect.
This process generates chondroid bone, an intermediate tissue between
bone and cartilage. It derives directly from cells like to chondrocytes as
a product of the transition from fibrous tissue to bone tissue. The process
occurs gradually and in consecutive form without invasion of vascular capillaries(15).
The presence of the protein in the first phases, specifically in the collagen
fibres surrounding the mesenchymal cell type suggests that OPN
is a necessary condition to facilitate the proliferation and migration of
preosteoblasts.(16)
The same condition is present in the matrix to make osteoblastic proliferation
in the trabecular surface possible.(17) The OPN which was surrounded
the oval cytoplasm cells contained in the matrix suggests that the
pre-osteoblasts synthesize them.
Between day 15 and 30 OPN was expressed in the osteoclasts and osteoblasts,
in the osteoid and in the ossification area. The immunomarcation
of OPN in the osteoblasts and in the osteoid showed differentiation of the
pre-osteoblasts in immature osteoblasts. OPN in the osteoid contributed
to the fixation of the osteoblasts and osteoclasts in the surfaces of spiculas
and trabecules and at the same time it made the biomineralization of the
extracellular matrix possible. With regard to the cartilaginous tissue immediate
to the ossification zone, the authors detected OPN in some of the
hypertrophic chondrocytes, as reported by Lian et al., (1993)(18) However,
it can be inferred that it is a process of transchondral ossification or cellular
transdifferentiation where the chondrocytes differentiate on a continuum
into osteoblasts.(19, 20, 21, 22,15,23) Recent investigations showed that the cellular
transdifferentiation comes from a continuous lineage of chondroblasts
which differentiate into osteoblasts(24,25,26) coming from cells called
osteoprogenitor chondrocytes (OC) which can generate a population of osteoblasts in vivo and in vitro.(27) This allowed the authors to infer that the
cells which marked OPN are OC synthesizing the non-collagen protein.
OPN made the cellular adhesion possible due to its interaction with
integrin αvβ3 and the transmembrane CD44 protein of the osteoblasts and
osteoclasts.(9,10) For this reason, the OPN synthesis of part of the mesenchymal
tissue conditions the initiation of the repair with the formation of
new bone which allows the adhesion of osteoblasts and intervenes in the
cellular differentiation.
The histological evidence did not establish that the DBM particles repaired
the defects by ostoconduction mechanisms. Instead, the mesenchymal
condensations that initiated ostogenesis were produced from them
The presence of osteoclasts at day 15 alludes to the fact that these recognized
the demineralized matrix and absorbed it exposing the BMP molecules
it contains. The evidence of positive chemotaxis that the MOD
particles exerted on the CM as well as the proliferation and differentiation
into chondrocytes and these into preosteoblasts, osteoblasts and osteocytes
indicate that DBM exerted osteoinduction in the repair site.(28)
The OD and IOD resulted in a useful tool to quantify the OPN immunosuppression.
In this way, the authors obtained objectively the
expression of density for each studied period and could establish statistically
significant differences that allowed them to infer the transcendence
of the expression.
The OD and IOD showed a marked increment at day 15 of treatment
to later diminish towards day 60 and it increased again at day 150. The
curves showed that the major metabolic OPN synthesis activity occurred
at day 15 and although it diminished towards day 60 there never
was total absence of OPN. The new increase of density observed at day
150 corresponded to the bone adaptive remodelling which became
more intense and therefore incorporated more minerals.
The variations in the arbitrary units in which the OD and the IOD
are expressed can be attributed to the fact that the OD in each period
represented the media that correspond to both the immunosignalling
of the bone matrix and the cells in which it expressed. In this sense, the
highest values corresponding to the IOD can be attributed to the fact
that the medias of the density were concentrated by square micron.
The graphic representations of the OD and the IOD had a similar behaviour
with a higher density of expression towards day 15 and then it
decreased slowly towards day 60, when it started to increase, although
with lower values than those registered at day 15. These variations
of density suggest that the greater metabolic activity was registered in
this period because of the cellular proliferation and of the expression of immature osteoblasts recently differentiated. On the other hand,
the Od and IOD of OPN synthesized by the CM were quantified.
The decrease in the expression of OPN between days 21 and 60 can
be interpreted as the result of the decrease in the cellular proliferation
followed by the decrease of differentiation of pre-osteoblasts in
osteoblasts, measured through the OD and IOD between days 21 and
60. The increase in the expression observed since day 60 to day 150
would correspond to the bone adaptive remodelling to the functional
member(8). established the role of OPN in the bone remodelling due to
the interrelation between osteoblasts and osteoclasts increasing the
biomineralization. This adaptive remodelling requires the increase of
minerals, for which a higher expression of OPN is required.
The present work offers a new view of the role of OPN in the repair
of experimental orthopedic bone defects treated with DBM. By means
of IHQ techniques quantified by OD and IOD, the authors established
the expression and intensity of manifestation of OPN during the period
of repair of the defects and their remodelling. Even though OPN
is not a protein that intervenes in the cellular differentiation, its expression
made proliferation and migration of preosteoblasts and osteoblasts
possible while the process of biomineralization of the new
bone matrix was on its way.
Table 1. Optic Density (OD) and Integrated Optic Density (IOD) of OPN
in each of the studied periods
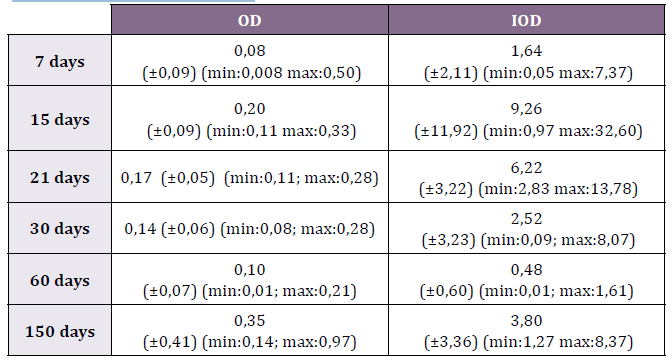
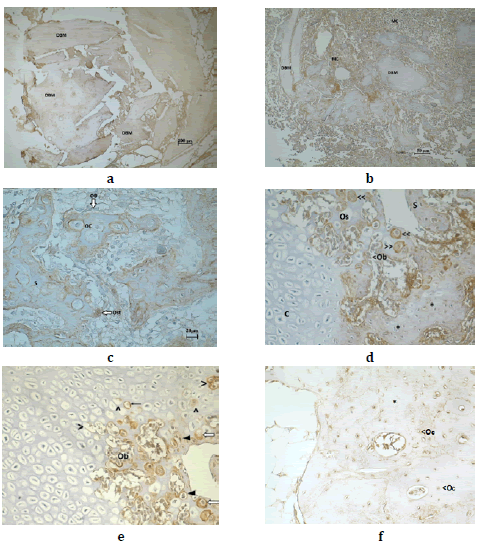
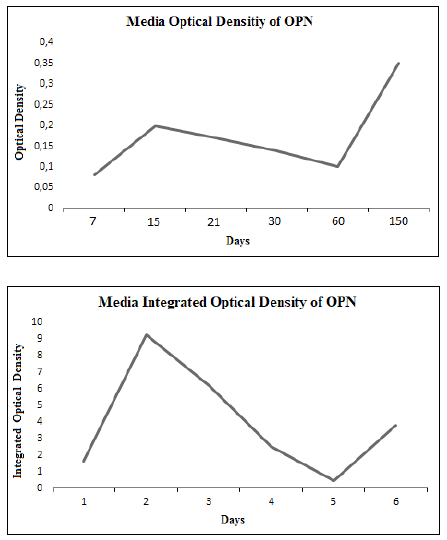
Fig. 2. Graphic representation of the media OD and IOD of OPN in the studied
period.
1. Urist, M. R. Bone formation by autoinduction. Science.1965; 150:893-899.
2. Colnot, C., D. M. Romero, S. Huang & J. A. Helms.Mechanism of action of demineralized bone matrix in the repair of cortical bone defects. Clinical Orthopaedic Related Research. 2005; 435:69-78.
3. Eppley, B. L., W. S. Pietrzak & M. W. Blanton. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. Journal of Craniofacial Surgery. 2005; 16:981-989.
4. McKee, M. D., C. M. Farach, W. T. Butler, P. V. Hauschka & A. Nanci. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HSglycoprotein) proteins in rat bone. Journal of Bone Mineral Research. 1993; 8:485–496.
5. Weber, G. F., S. Ashkar, Glimcher, M. J. & H. Cantor. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996; 271:509-512.
6. Chellaniah, M. A, K. A. Hruska. The integrin alpha(v)beta(3) and CD44 regulate the
actions of osteopontin on osteoclast motility. Calcified Tissue International. 2003;
72:197–205.
7. Zhu, B., K. Suzuki, H. A. Goldberg, S. R. Rittling, D. T. Denhardt, C. A. McCulloch & J. Sodek. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. Journal of Cell Physiology. 2004; 198: 155–167.
8. Uemura, T., A. Nemoto, Y. K. Liu, H. Kojima, J. Dong, T. Yabe, T. Yoshikawa, H. A. Ohgushi, T. Ushida & T. Tateishia. Osteopontin involvement in bone remodeling and its effects on in vivo osteogenic potential of bone marrow-derived osteoblasts/porous hydroxyapatite constructs. Materials Science and Engineering. 2001; 17:33–36.
9. Sodek, J., B. Ganss & M. D. McKee. Osteopontin. Critical Review in Oral Biology and Medicine. 2000; 11: 279–303.
10. Lesley, J., R. Hyman, P.W. Kincade. Hyaluronan binding by cell surface CD44. Journal of Biological Chemistry. 2000; 275:26967-26975.
11. Hollinger, J. O. & J. C. Kleinschmidt. The critical size defect as an experimental model to test bone repair methods. Journal of Craniofacial Surgery. 1990; 1:60-68.
12. Audisio, S. A., P. G. Vaquero, P. A. Torres, E. C., L. N. Ocampo, V. Ratusnu, A. L., Cristofolini, C. I. Merkis. Obtención, caracterización y almacenamiento de matriz ósea desmineralizada. Revista de Medicina Veterinaria. 2014; 95:27-34.
13. Vasconcellos, A., C. Cisternas & M. Paredes. Estudio inmunohistoquímico comparativo del receptor de estrógeno en tejido endometrial de ovejas razas Texel y Araucana. International Journal of Morphology. 2014; 32:1120-1124.
14. Di Rienzo, J.A., F. Casanoves, M.G. Balzarini, L. Gonzalez, M Tablada & C.W. Robledo. InfoStat versión Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. 2010.
15. Yasui, N., M. Sato, T. Ochi, T. Kimura, H. Kawahata, Y. Kitamura & S. Nomura. Three modes of ossification during distraction osteogenesis in the rat. Journal of Bone and Joint Surgery.1997; 79,:824-830.
16. Radomisli, T. E., D. C. Moore, H. J. Barrach, H. S. Keeping & M. G. Ehrlich.. Weight-bearing alters the expression of collagen types I and II, BMP 2/4 and osteocalcin in the early stages of distraction osteogenesis. Journal of Orthopedic Research. 2001; 19:1049-1056.
17. Jang, J. H. & Kim J.H. Improved cellular response of osteoblast cells using recombinant human osteopontin protein produced by Escherichia coli. Biotechnology Letter. 2005; 27:1767–1770.
18. Lian, J. B., M. D. McKee MD, Todd AM, Gerstenfeld LC. Induction of bone-related proteins, osteocalcin and osteopontin, and their matrix ultrastructural localization with development of chondrocyte hypertrophy in vitro. Journal of Cellular Biochemistry. 1993; 52:206–219
19. Silbermann, M., D. Lewinson, H. Gonen, M. A. Lizarbe & K. von der Mark K. In vitro transformation of chondroprogenitor cells into osteoblasts and the formation of new membrane bone. The Anatomy Record. 1983; 206: 373-383.
20. Moskalewski, S. & J. Malejejcyk.. Bone formation following intrarenal transplantation of isolated murine chondrocytes: chondrocyte – bone cell differentiation. Development. 1989; 107: 473-480.
21. Thesingh, C. W., Groot, C. G. & A. M. Wassenaar.Transdifferentiation of hypertrophic chondrocytes into osteoblasts in murine fetal metatarsal bones, induced by co-cultured cerebrum. Bone and Mineral Research.1991; 12:5-40.
22. Descalzi Cancedda, F., C. Gentili, P. Manduca & R. Cancedda. Hypertrophic chondrocytes undergo further differentiation in culture. Journal of Cellular Biology. 1992; 117:427-435.
23. Enishi, T., K. Yukata, M. Takahashi, R. Sato, K. Sairyo K, N. Yasui. Hypertrophic chondrocytes in the rabbit growth plate can proliferate and differentiate into osteogenic cells when capillary invasion is interposed by a membrane filter. 2014; PLoS ONE 9, e104638.
24. Yang, G., L. Zhu, N. Hou, Y. Lan, X. M. XM, B. Zhou, Y. Teng & X. Yang. Osteogenic fate of hypertrophic chondrocytes. Cell Research. 2014a;24:1266-1269.
25. Yang, L., K. Y. Tsang, H. C. Tang, D. Chan & K. S. Cheah.. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proceedings of National Academy Science. 2014b; 111:12097-12102.
26. Zhou, X., K. von der Mark, S. Henry, W. Norton, H. Adams, B. de Crombrugghe.. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genetics. 2014; 10, e1004820.
27. Jung, P., M. Gebhardt, S. Golovchenko, F. Perez-Branguli, T. Hattori, C. Hartmann, X. Zhou, B. deCrombrugghe, M. Stock, H. Schneider & K. von der Mark. Dual pathways to endochondral osteoblasts: a novel chondrocytederived osteoprogenitor cell identified in hypertrophic cartilage. Biology Open. 2015; 4,608–621.
28. Kawakami, T. Immunohistochemistry of BMP induced heterotopic osteogenesis. Journal of Hard Tissue Biology.2001; 10:73-76.
Fecha de recepción artículo original: 09-08-2018
Fecha de aceptación para su publicación: 06-02-2019