DOI: http://dx.doi.org/10.19137/cienvet202224106
![]() Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No Comercial-
Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons.org.ar/licencias.html
Esta obra se publica bajo licencia Creative Commons 4.0 Internacional. (Atribución-No Comercial-
Compartir Igual) a menos que se indique lo contrario, http://www.creativecommons.org.ar/licencias.html
COMUNICACIONES CORTAS
Effect of rumen protected thiamine on blood
concentration of beta-hydroxyl butyrate in
postpartum Holstein cows: a pilot study
Efecto de la tiamina protegida en rumen sobre la
concentración sanguínea de beta hidroxibutirato
en vacas Holstein posparto: un estudio piloto
Efeito da tiamina protegida no rúmen sobre a
concentração sanguínea de beta-hidroxil butirato
em vacas holandesas pós-parto: um estudo piloto
Melendez P1, Riquelme P2, Reyes C2
1 School of Veterinary Medicine, Texas Tech University, Amarillo, TX 79106, USA.
2 Pahuilmo Dairy Farm, Mallarauco, Chile.
Correspondence: pedro.melendez@ttu.edu
Abstract: A development of a rumen bypass product containing thiamine
might be a valuable alternative on the prevention of ketosis in dairy
cattle. The objective of this pilot study was to determine the effects
of rumen protected thiamine (RPT) on blood beta-hydroxyl butyrate
(BHB) in Holstein postpartum cows. The study was conducted on a
dairy herd with 650 cows, randomly assigned to a treatment group
(T, n=20) receiving daily for 10 days postpartum, orally, 60 g rumen
protected thiamine, and a control group (C, n=20), receiving a placebo.
Blood samples were collected on days 3, 7, and 10 after calving. At
day 3 and 10 postpartum BHB levels were similar between groups; however, at day 7, BHB concentrations were different (0.57 and 0.83
mmol/L for T and C respectively, P ≤0.05). It is concluded that a rumen
protected thiamine oral product decreased the blood concentrations
of BHB during the first 10 days postpartum. Based on this pilot study,
this additive deserves further investigation to elucidate its potential
mechanism of physiological action as a ketosis preventive agent.
Keywords: thiamine, ketosis, beta-hydroxyl butyrate, Holstein,
postpartum, cattle
Resumen: El desarrollo de un producto de sobrepaso del rumen que contenga
tiamina podría ser una alternativa valiosa para la prevención de la
cetosis en el ganado lechero. El objetivo de este estudio piloto fue determinar
los efectos de una tiamina protegida de sobrepaso del rumen
(RPT) sobre el beta hidroxibutirato (BHB) en sangre en vacas Holstein
posparto. El estudio se realizó en un hato lechero con 650 vacas, asignadas
aleatoriamente a un grupo de tratamiento (T, n = 20) que recibió diariamente durante 10 días después del parto, por vía oral, 60 g
de tiamina protegida de sobrepaso del rumen y un grupo de control (C,
n = 20), recibiendo un placebo. Se recolectaron muestras de sangre los
días 3, 7 y 10 posparto. En el día 3 y 10 después del parto, los niveles
de BHB fueron similares entre los grupos; sin embargo, en el día 7, las
concentraciones de BHB fueron diferentes (0.57 y 0.83 mmol / L para T
y C respectivamente, P ≤0.05). Se concluye que un producto oral de tiamina
protegido en el rumen disminuyó las concentraciones sanguíneas
de BHB durante los primeros 10 días posparto. En base a este estudio
piloto, este aditivo merece una mayor investigación para dilucidar su
mecanismo potencial de acción fisiológica como agente preventivo de
la cetosis.
Palabras clave: tiamina, cetosis, beta hidroxibutirato, Holstein,
posparto, ganado lechero
Resumo: O desenvolvimento de um produto derivado do rúmen contendo
tiamina pode ser uma alternativa valiosa na prevenção da cetose em
gado leiteiro. O objetivo deste estudo piloto foi determinar os efeitos
da tiamina protegida no rúmen (RPT) sobre o beta-hidroxil butirato
(BHB) sanguíneo em vacas holandesas no pós-parto. O estudo foi conduzido em um rebanho leiteiro com 650 vacas, aleatoriamente designadas
a um grupo de tratamento (T, n = 20) recebendo diariamente
por 10 dias pós-parto, por via oral, 60 g de tiamina protegida no rúmen
e um grupo de controle (C, n = 20 ), recebendo um placebo. Amostras
de sangue foram coletadas nos dias 3, 7 e 10 pós-parto. Nos dias 3 e
10 pós-parto, os níveis de BHB foram semelhantes entre os grupos;
entretanto, no dia 7, as concentrações de BHB foram diferentes (0,57
e 0,83 mmol / L para T e C respectivamente, P ≤ 0,05). Conclui-se que
um produto oral com tiamina protegida no rúmen diminuiu as concentrações
sanguíneas de BHB durante os primeiros 10 dias pós-parto.
Com base neste estudo piloto, este aditivo merece uma investigação
mais aprofundada para elucidar o seu potencial mecanismo de ação
fisiológica como agente preventivo da cetose.
Palavras-chave: tiamina, cetose, butirato de beta-hidroxila,
Holstein, pós-parto, gado
During the transition period the dairy cow must undergo severa
adaptive changes, including the reduction of dry matter intake, rumen
adaptation to diets high in starch, decrease the risk and severity of hypocalcemia
oxidative stress and immunosuppression. Consequently, the
cow may become more susceptible to several diseases, which represent
high economic losses for the dairy industry (1, 3). Peripartum diseases are
closely related to each other, where cows with ketosis, for example, are
more likely to develop displacement of the abomasum, impaired fertility
and reduced milk production. Therefore, prevention of ketosis indirectly
reduces the risk of other postpartum disorders (4, 7).
Ketosis is a metabolic disease associated with the typical negative
energy balance experienced by dairy cows during the postpartum period.
The mechanism of exacerbated ketogenesis lies in the low dry
matter intake that cows have around parturition and the sudden onset
of milk production just after calving. As a result, the cow begins to
mobilize adipose tissue in the form of non-esterified fatty acids. At the
same time, glucose is redirected to the mammary gland and spared to
produce lactose. Non-esterified fatty acids are taken by the liver and
oxidized to acetyl coA. Because of lack of glucose (used for the milk
synthesis), oxaloacetate production is reduced, then acetyl-co A cannot
continue its oxidation process in the Krebs cycle, resulting in an increase
rate of ketogenesis. Ketone bodies are the acetoacetate, acetone and ß-hydroxybutyrate (BHB), being the last the most important to be
tested in blood. (6, 8).The clinical manifestation of ketosis is characterized
by reduced dry matter intake and milk production, which may be
accompanied by the presence of neurological signs, while the subclinical
condition apparently shows no evident signs and is established
when blood BHB is ≥ 1.2 mmol/L. (4,6). The reported cost of subclinical
ketosis is widespread, ranging from US$ 77 to US$ 289 per case in the
United States (3).
Ketosis has been a focus of intense research during the last decades. (3, 5-7). In fact, studies carried out by the Cornell University in the USA,
determined that cows with prepartum blood levels of non-esterified
fatty acids > 0.3 mEq/L and postpartum BHB > 1.2 mmol/L were 2 to 4
times more likely to experience periparturient diseases, reduce pregnancy
rate and decrease milk yield than normal cows. (5, 7). As mentioned
above, ketosis occurs in response to the lack of gluconeogenic
precursors. (8). Therefore, many additives, including niacin, monensin,
propylene glycol, glycerol, and propionate have been studied as potential
nutritional strategies to prevent ketosis in dairy cows (2). However,
certain key enzymes in the intermediate metabolism at the hepatocyte
level deserve much more attention. Thiamine pyrophosphate is the
physiologically active form of vitamin B1, participating as a cofactor of
key enzymes of intermediate metabolism, such as pyruvate dehydrogenase.
This is a multi-enzyme complex, where under certain anaerobic
conditions it decarboxylates pyruvate to acetyl Co-A. Thiamine also
participates within the citric acid cycle in the decarboxylation of alpha
ketoglutarate to succinyl Co-A, favoring the oxidation of glucose to obtain
ATP (8). All these reactions, in theory, should favor the formation of
oxaloacetate that assists the entry of acetyl Co-A into the Krebs cycle,
avoiding ketogenesis (8). However, the contribution of thiamine in the
diet of dairy cows, if not protected from the action of microorganisms
of the rumen, may not have the desired effect. Therefore, the technological
development of a rumen bypass product containing thiamine
pyrophosphate (RPT) might be a valuable alternative on the prevention
of ketosis in dairy cattle. Thus, the hypothesis of this pilot study
was that the supplementation of a RPT in dairy cows reduces the concentration
of BHB in the blood. Hence, the objective of the present investigation
was to evaluate the effect of an oral RPT supplemented during
the first 10 days postpartum on the blood concentrations of BHB
and accumulated milk production up to 100 days in milk in Holstein
cows.
The study was conducted under the ethical guidelines of Chilean government for the use of animals in research.
DAIRY AND ANIMALS
This is a pilot study with the intend of using the results for further
controlled large-scale investigations. The study was carried out on a
Holstein dairy (Pahuilmo dairy farm), with 1,300 total cattle, and 650
milking cows in the central area of Chile (Latitude: -33.58. Longitude:
-71.12). The mean annual precipitation and lowest and highest temperature
were 235 mm, 2.2 ° C and 25 ° C, respectively [9]. Cows were
confined in an open barn free-stall system with sand beds, shade, and
concrete floor. Cows were milked 3 times a day, in a rotatory parlor,
with a Mature Equivalent 305-day milk yield of 12,000 kg. Milk yield
was recorded daily in a computerized milking record system (Afimilk,
Kibbutz Afikim, 1514800, Israel).
Cows were fed a total mixed ration three times a day. Cows were
dried-off between 45 and 70 days before expected parturition. At 4
weeks before expected parturition, cows were moved to a prepartum
lot and were fed a total mixed ration with a dietary cation-anion difference
of -80 mEq/kg dry matter to prevent clinical hypocalcemia.
Prepartum total mixed ration was fed twice a day. Cows calved in individual
maternities and moved to a postpartum group until 21 days
in milk. Calf was removed from the dam immediately after birth. Diets
were formulated to meet or exceed the nutritional requirements of
the Cornell Net Carbohydrate and Protein System (CNCPS) (10), using a
commercial software (NDS, RUM&N Sas, Reggio Emilia, Italy).
During the postpartum period cows were subjected during the first
10 days in milk to a health monitoring protocol every other day, measuring
rectal temperatures, presence of retained fetal membranes, abnormal
uterine discharges, presence of abnormal milk for the diagnosis
of clinical mastitis, evaluation of left paralumbar fossa to determine
rumen fill and the presence of left displacement of abomasum or any
other evident clinical condition. If cows were diagnosed with any pathological
disorder, they were treated according to standard operating
procedures established by the farm veterinarian.
Subsequently, at 38 ± 3 days in milk cows were subjected to a synchronization
of ovulation protocol (Presynch-Ovsynch) and Timed-
Artificial Insemination or breeding on heat detection. Pregnancy was
diagnosed by ultrasound at 34 ± 3 days post service.
EXPERIMENTAL DESIGN
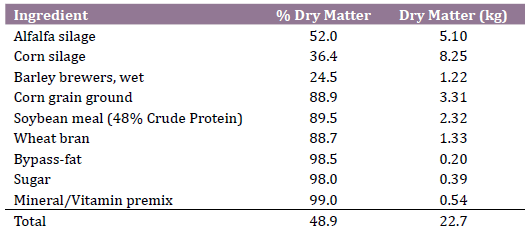
In order to find a difference in blood BHB concentration of 0.2 mmol / L (Standard Deviation = 0.20) between a treated group (1.0 mmol/L) and a control group (1.2 mmol/L; cut-off value for subclinical ketosis (6, 7), with 95% of confidence and 80% of power, a minimum sample size of 15 cows per group was calculated. Animals were randomly assigned at parturition to a treatment group (n=20) receiving 60 g/day of a rumen-bypass thiamine pyrophosphate (Glukogen, Nutritech, Agua Termal 100 Col., Agua Blanca Sur, Zapopan Jalisco, Mexico) dissolved in 100 ml of water, and offered orally with a syringe after the morning milking from day 1 to 10 postpartum. The control group (n=20) received a placebo of 100 ml of water. Both treated and control cows were housed in the same postpartum pen, received the same total mixed ration and were exposed to the same environmental and management conditions. Ingredients and nutritional composition of diets are shown in Table 1.
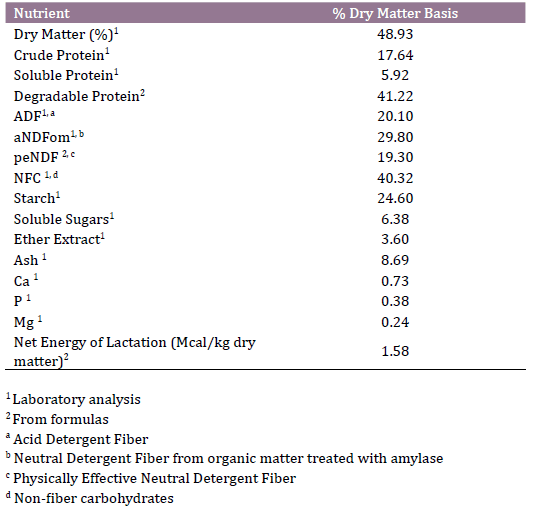
Table 1. Ingredients and nutritional composition of postpartum total mixed
ration (kg/d, dry matter basis)
BLOOD SAMPLING AND MILK PRODUCTION
After the morning milking, experimental cows were sorted out by an
electronic computerized gate system and then placed in a chute with
a headlock for treatment and placebo oral supplementation and blood
collection. Samples were obtained from the tail plexus and were collected
at 3, 7, and 10 days postpartum to assess BHB concentrations using
a portable meter device (FreeStyle Optium®, Abbott Diabetes Care Inc.,
Alameda, CA). The handheld device has a sensitivity of 94.8% (95% CI:
92.6-97.0), and a specificity of 97.5% (95% CI: 96.9-98.1) (11). After finishing
the daily treatment supplementation and blood sampling protocol,
cows were returned to their group. Treatment and blood sampling
lasted for no more than 5 minutes per cow. Body condition score at
calving was measured by the same evaluator using a scale 1-5 (12). Milk
production was measured and recorded daily, and cumulative milk production
up to 100 days in milk was analyzed.
STATISTICAL ANALYSIS
Data analysis was performed using the software SAS 9.4 (13). The
concentrations of blood BHB were analyzed using a mixed model
analysis for repeated measures, considering the effect of treatment
(RPT, control), day of sampling (3, 7, 10 days postpartum), lactation
(primiparous, multiparous), body condition score at calving and their
potential interactions as explanatory variables. The cow was considered
as random effect nested within treatment and the interaction
treatment x day of sampling was considered the most important variable
of the model, since it measures the parallelism of the BHB concentration
curves between both groups over time. The best model fitting
was obtained using the Schwarz Bayesian Criterion, according to the
best covariance structure matrix between the observations (auto-regressive,
uncorrelated, and symmetric) (14). The level of significance
was established at P ≤ 0.05. A tendency was considered with a P-value
between 0.15 and 0.05. Cumulative milk production up to 100 days in
milk was compared between groups by an analysis of variance, considering
as independent effects the parity number; body condition score
at calving, and treatment, using the PROC GLM of SAS 9.4 (13). Leas
square means were reported.
Mixed model for repeated measures considered as significant variables
the effect of day postpartum (P = 0.0022) and the interaction of
treatment x day (P = 0.037). Lactation and body condition score at calving
were non-significant variables. (Table 2). Covariance parameters demonstrated that the auto-regressive covariance structure was the
best for goodness of fit. This indicates that the BHB concentrations
curves between both groups were non-parallel and therefore differed
over time (Figure 1). BHB concentrations were similar between
groups at day 3 postpartum (> 0.7 mmol/L); however, at day 7 postpartum
BHB concentrations decreased drastically in the RPT group
(< 0.6 mmol/L) while increased drastically in the control group (> 0.8
mmol/L). At day 10 postpartum, both groups had similar BHB concentrations
(< 0.6 mmol/L).
Cumulative milk production at 100 days in milk were 4626.6 kg for
the control group and 5208.8 kg for the treated group, tending to be
different (Standard Deviation = 535 kg) (P = 0.11).
Figures and Tables
Table 2. T Mixed model for repeated measures. Type III Tests, F-statistics and
probabilities for fixed effects for blood BHB concentrations (mmol/L) in cows
supplemented with rumen protected thiamine.
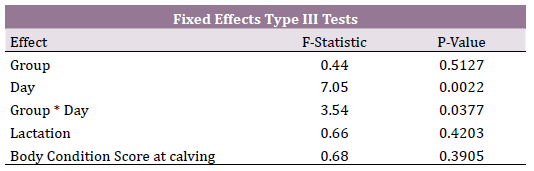
Figure 1. Blood concentrations of BHB (mmol/L) in cows treated with rumen
protected thiamine and controls at day 3, 7 and 10 postpartum. Interaction day
x treatment (P =0.037). Higher concentration of BHB at day 7 postpartum for
control than treatment group (P < 0.001).

Ketosis is a typical metabolic disorder of dairy cows during the early
postpartum period. It is an alteration associated to the metabolism
of glucose and fatty acids that is closely related to the typical negative
energy balance during the onset of lactation. Therefore, the highest
incidence of ketosis occurs within the first 7 days postpartum
(5, 6). According to the results of the present investigation, our hypothesis
was accepted since BHB concentrations were lower in the treated
group than the control group, especially at 7 days postpartum.
We have found no scientific publication reporting the impact of any
product based on any potential oral rumen protected thiamine on the
levels of BHB in Holstein dairy cows.
Although, in both groups, BHB concentrations were below the cutoff
value to categorize cows as having subclinical ketosis (> 1.2 mmol /
L) (6, 7) the reduction of BHB in the treated group suggests a factual benefit
for the dairy cow. Higher production of ketone bodies can inevitably
lead to a vicious cycle of lower dry matter intake and higher BHB
production in the liver, which is undesirable for cows during the early
postpartum period (15). Ketosis is a costly disease that is strongly associated,
as a risk factor, to displacement of the abomasum. In addition, displacement of the abomasum is a periparturient disease with the
highest economic cost reported for dairy cattle in the US (US$ 650) (3),
consequently, by preventing a case of ketosis, the economic benefit for
the dairy producer is evident and extremely important.
Humans with severe diabetic keto-acidosis along with encephalopathy
developed concomitantly a severe deficiency of thiamine in the
blood. When patients received an intravenously thiamine-based product,
they showed an immediate positive response. The authors of that
report suggested that the thiamine deficiency had a very consistent
association with the ketosis and encephalopathy in the patients (16). In
another prospective study, 15 human patients out of 22 with diabetic
keto-acidosis had a blood thiamine deficiency. This study concluded
that thiamine deficiency is common in children with diabetic ketosis,
and that it worsens when patients are treated with insulin. Therefore,
when metabolic acidosis persists despite adequate treatment of diabetic
keto-acidosis, other factors such as thiamine deficiency should
be considered (17). Undoubtedly, thiamine has a very consistent association
with BHB levels in blood of human diabetic patients, and this
relationship may also be present in transition dairy cows, where in
the present study RPT supplementation in postpartum cows reduced
the concentration of BHB in blood. One of the major drawbacks of this
pilot study was the no evaluation of thiamine in blood. Unfortunately,
this assay was not feasible to be carried out, but it is considered essential
for further studies to evaluate thiamine levels in blood to demonstrate
the potential rumen bypass effect.
Thiamine participates as a cofactor of the enzyme pyruvate dehydrogenase,
alpha-ketoglutarate dehydrogenase and transketolase,
where it also participates within the Krebs cycle in the decarboxylation
of alpha-ketoglutarate to succinyl-CoA (8). Consequently, since al
these reactions favor the formation of oxaloacetate and assist the entry
of Acetyl Co-A into the Krebs cycle, it is reasonable to suggest that
the supplementation of RPT may play certain role in the reduction of
ketogenesis.
In relation to milk production, the group treated with RPT tende
to have a higher milk yield than the control group (P=0.11) with
approximately 500 kg more milk accumulated during the first 100
days in milk. To some extent, similar results were found in a study that
reported 3 experiments with thiamine supplementation. In this investigation,
thiamine was not rumen bypass and only 1 out of the 3 experiments
found higher milk yield. The third experiment found lower
milk yield and fat content in the thiamine group when compared with
the control group (18). In addition, in a study conducted in Egypt, cows supplemented with 340 mg of non-protected thiamine increased milk
production, fat and protein yields. Interestingly, serum thiamin concentration
was significantly higher in the supplemented group than
the control diet, meaning part of the thiamine bypassed the rumen (19).
These studies did not evaluate BHB concentration in blood. Ketosis
can be associated with either low or high milk production (3-7). If thiamine
has a positive effect on liver function, making intermediate metabolism
more efficient, especially at the level of Krebs cycle, it supports
the findings that the synthesis of ketone bodies may be reduced and
there might be greater gluconeogenic precursors available to favor
milk production.
B-complex vitamins are supposed to be synthesized in sufficient
quantities by rumen microorganisms and deficiencies are rarely observed
in ruminants. Nevertheless, over the last years, numerous
studies have reported that, under certain conditions, subclinical deficiencies
of B vitamins may occur in high producing dairy cows. Both,
rumen synthesis and degradation of some B vitamins, including thiamine,
have been observed in cattle (20). It is well-known that the excess
of dietary sulfur and starch, that may induce rumen acidosis, may
trigger thiamine deficiency in ruminants (21). Consequently; thiamine
supplementation may have beneficial effects in dairy cows, increasing
milk yields and solids components (19). In the lights of the present results,
further experimental designs are warranted to confirm that RPT
is truly rumen bypass, by measuring it in blood, and by evaluating several
other intermediary metabolites that participate in the process of
ketogenesis, fats, and carbohydrate metabolism. In addition to its role
in carbohydrate and energy metabolism, the effects of thiamine on cell
regulation, immune function, and oxidative stress should be evaluated.
In addition, the additive should be tested in a way that is easy to
administer in feed, ideally blended in a total mixed ration, since the
administration via oral with an individual syringe is impractical and
difficult to carry out in commercial conditions of dairy operations. For
the prevention of ketosis, under Chilean conditions, daily cost of supplementation
of propylene glycol is US$ 0.54, monesin is US$ 0.25,
and for the current tested product (RPT) is US$ 0.24. If the use of this
additive proves to be effective, rumen protected thiamine might be
considered as an innovative nutritional strategy to improve thiamine
status, reduce ketogenesis, increase milk production and improve profitability
of high-producing lactating dairy cows.
This pilot study suggests that a potential rumen protected thiamine
product decreases the blood concentrations of BHB during the early
postpartum period of Holstein cows. The results of this investigation
open a window to further evaluate a potential impact of a vitamin B1
rumen bypass product to prevent ketosis and improve milk production
in lactating dairy cows.
1. Drackley JK. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci 1999, 82, 2259-2273.
2. Melendez P Risco CA. In Reference Module in Food Sciences. Reproduction, Events and Management Pregnancy: Periparturient Disorders. 1st ed.; Geoffrey, S., Ed.; Elsevier. Academic Press, 2016; https://doi.org/10.1016/B978-0-08-100596-5.01048-9
3. Liang D, Arnold LM, Stowe CJ, Harmon RJ, Bewley JM. Estimating US dairy clinical disease costs with a stochastic simulation model. J Dairy Sci 2017, 100, 1472-1486.
4. Melendez P, Risco CA. Management of transition cows to optimize reproductive efficiency in dairy herds. Vet Cl North Am Food Anim Prac 2005, 21, 485-501.
5. Ospina PA, Nydam DV, Stokol T, Overton TR. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and beta-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J Dairy Sci 2010, 93, 3595-3601.
6. McArt JAA, Nydam DV, Oetzel GR. Epidemiology of subclinical ketosis in early lactation dairy cattle. J Dairy Sci 2012, 95, 5056-5066.
7. Ospina PA, McArt JAA, Overton TR, Stokol T, Nydam DV. Using nonesterified fatty acids and β-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. Vet Clin North Am. Food Anim Pract 2013, 29, 387-412.
8. Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 7th ed.; Macmillan Learning, 300 American Metro Blvd, Suite 140, Hamilton, NJ 08619, USA, 2017; pp. 601-630.
9. Boletín Agro-Meteorológico Decadal, Santiago: Chile. Available online: http://www. meteochile.gob.cl/PortalDMC-web/index.xhtml
10. Van Amburgh ME, Collao-Saenz E, Higgs R, Ross DA. Recktenwald, E.B.; Raffrenato, E.; Chase, L.E.; Overton, T.R.; Mills JK, Foskolos A. The Cornell Net Carbohydrate and Protein System: Updates to the model and evaluation of version 6.5. J Dairy Sci 2015, 98, 6361-6380.
11. Tatone EH, Gordon JL, Hubbs J, LeBlanc SJ, DeVries TJ, Duffield TF. A systematic review and meta-analysis of the diagnostic accuracy of point-of-care tests for the detection of hyperketonemia in dairy cows. Prev Vet Med 2016, 130, 18-32.
12. Ferguson JD, Galligan DT, Thomsen N. Principal descriptors of body condition score in Holstein cows. J Dairy Sci 1994, 77, 2695-2703.
13. SAS INSTITUTE. SAS/STAT Software: Change and enhancements through release 9.4 for Windows. SAS Institute Inc., Cary, NC, USA. 2013.
14. Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci 1998, 76, 1216-1231.
15. Overton TR, McArt JAA, Nydam DV. A 100-Year Review: Metabolic health indicators and management of dairy cattle. J Dairy Sci 2017, 100, 10398-10417.
16. Clark JA, Burny I, Sarnaik AP, Audhya TK. Acute thiamine deficiency in diabetic ketoacidosis: Diagnosis and management. Pediatr Crit Care Med 2006, 7, 595-599.
17. Rosner EA, Strezlecki KD, Clark JA, Lieh-Lai M. Low thiamine levels in children with type 1 diabetes and diabetic ketoacidosis: a pilot study. Pediatr Crit Care Med 2015, 16, 114-118.
18. Shaver RD, Bal MA. Effect of dietary thiamin supplementation on milk production by dairy cows. J Dairy Sci 2000, 83, 2335-2340.
19. Kholif AM Hanafy MA, El-Shewy A, Gawad MHA, Farahat ESA. Effect of supplementing rations with thiamin and/or sodium bicarbonate on milk yield and composition of lactating cows. Egyptian J Nut Feeds 2009, 12, 187-195.
20. Girard CL, Graulet B. Methods and approaches to estimate B vitamin status in dairy cows: Knowledge gaps and advances. Methods 2021, 186, 52-58
21. Pan X, Nan X, Yang L, Jiang L, Xiong B. Thiamine status, metabolism and application in dairy cows: a review. Br J Nutr 2018, 120, 491-499.
Fecha de recepción: 10/09/2021
Fecha de aceptación para su publicación: 24/11/2021